Jørgensen’s Organocatalysts

Product Manager:Nick Wilde
Introduction
Professor Karl Anker Jørgensen and his research team have synthesized (R)- and (S)-α,α-bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether compounds, which excel as chiral organocatalysts for the direct and asymmetric α-functionalization process of aldehydes.
In the realm of asymmetric synthesis, this stereoselective functionalization marks a pivotal advancement. Jørgensen's diarylprolinol silyl ether catalysts have demonstrated remarkable proficiency in catalyzing diverse bond-forming reactions, encompassing C-C, C-N, C-O, C-S, and C-Hal linkages, with both high yields and unparalleled enantiomeric excesses.

Figure 1. pyrrolidinemethanol trimethylsilyl 1
Representative Applications
α-Amination
The utilization of Jørgensen's organocatalyst for direct α-amination between aldehydes and azodicarboxylates at ambient temperatures has been reported to rival the enantioselectivities attained with L-proline as a catalyst. Intriguingly, despite the identical absolute configuration of the catalyst, the aminated products exhibit an inverted absolute configuration, attributed to steric hindrance effects, underscoring the unique reactivity of this organocatalytic system.

Figure 2. α-Amination 2
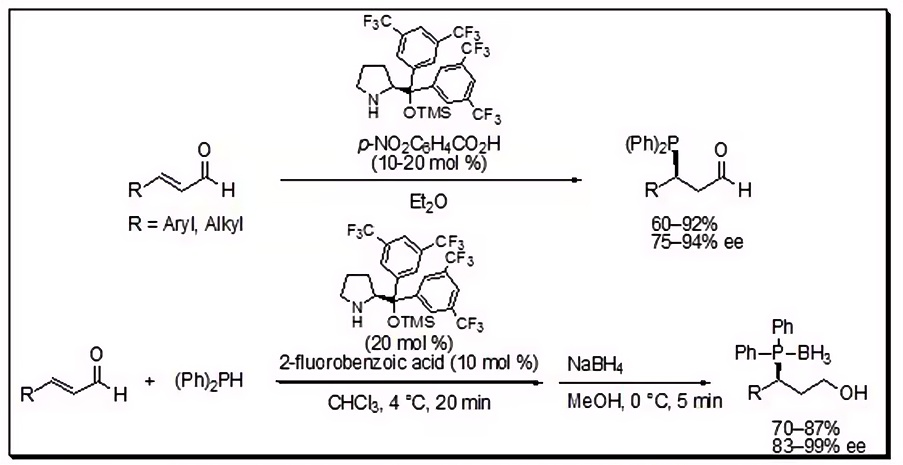
Domino Conjugated Nucleophilic Addition-Electrophilic Amination
Jørgensen's innovative one-pot, multicomponent domino reaction sequence, harnessing conjugated nucleophilic addition followed by electrophilic amination, offers a streamlined pathway to highly functionalized molecules. This methodology yields 1,2-aminothiol derivatives with exceptional enantioselectivities exceeding 99% ee. The gentle sulfur nucleophile initiates the process by engaging with an iminium ion intermediate, derived from an α,β-unsaturated aldehyde. Subsequently, the electrophile, an azodicarboxylate, seamlessly adds to the enamine intermediate, furnishing nearly enantiomerically pure products when catalyzed by 2-[bis(3,5-bistrifluoromethylphenyl)trimethylsilanyloxy-methyl]pyrrolidine, showcasing the power of this efficient and selective strategy.
Figure 3.Domino Conjugated Nucleophilic Addition 3
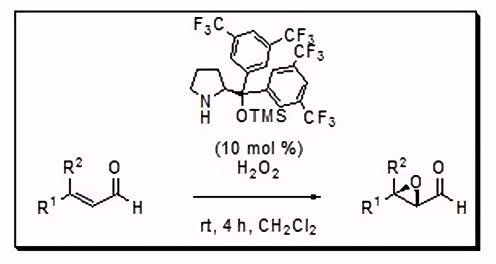
Asymmetric Epoxidation
Expanding upon direct α-functionalization achievements, Jørgensen groundbreakingly disclosed the first organocatalytic asymmetric epoxidation of α,β-unsaturated aldehydes, employing his sterically hindered chiral pyrrolidine derivative under eco-friendly conditions, notably utilizing hydrogen peroxide as the oxidant. This methodology successfully converted a diverse array of substituted enals into their corresponding α,β-epoxy aldehydes, achieving yields surpassing 90%, diastereoselectivities of up to 98:2, and remarkable enantioselectivities of up to 98%, marking a significant advancement in the field of asymmetric catalysis.
Figure 4.Asymmetric Epoxidation 4
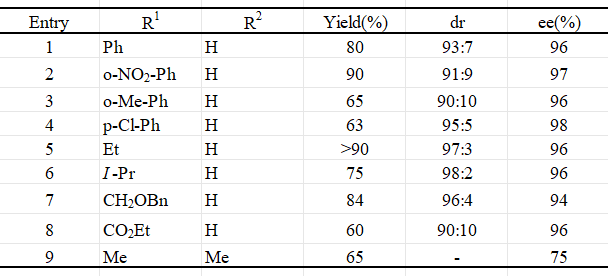
Asymmetric Hydrophosphinylation
Recently, Melchiorre and Córdova have concurrently pioneered the groundbreaking organocatalytic approach for asymmetric hydrophosphinylation of α,β-unsaturated aldehydes, enabling a direct synthesis route to β-phosphine aldehydes with remarkable enantiomeric excess. Notably, this organocatalytic method circumvents the issue of product inhibition often encountered in metal-catalyzed systems, where the phosphorus atom's coordinating capability can hinder the process. Furthermore, with minimal effort, the chiral phosphine products can be transformed into invaluable bidentate P-ligands, which are essential components in metal-catalyzed enantioselective reactions.
Figure 5.Asymmetric Hydrophosphinylation 5,6
Reference
1. Franzén, J. et al. J. Am. Chem. Soc. 2005,127,18296. https://doi.org/10.1021/ja056120u
2. Marigo, M: Jørgensen, K. A. α-Heteroatom Functionalization. In Enantioselective Organocataysis, Dalko, P. L, Ed.; Wiley-VCH;Weinheim, 2007; Chapter 2.2. https://doi.org/10.1039/b517090g
3. Marigo,M.et al.J.Am.Chem.Soc.2005,127,15710. https://doi.org/10.1021/ja055291w
4. Marigo,M. et al.J. Am. Chem. Soc. 2005, 127, 6964 .
https://doi.org/10.1021/ja051808s
5. Carlone,A.et al. Angew. Chem.,Int Ed. 2007 46,4504. https://doi.org/10.1002/anie.200705523
6. Ibrahem, I.et al. Angew.Chem, Int. Ed. 2007 46,4507. https://doi.org/10.1002/anie.200700916
For more information, visit our website: www.aladdinsci.com.