Light-emitting Polymers
Product Manager
Sandra Forbes
Introduction
Conjugated polymers featuring extended p-electron delocalization exhibit properties akin to processable organic "metals" when doped and function as semiconducting materials in their neutral, undoped state. Numerous undoped polymers demonstrate strong photoluminescence (PL) within the visible and near-infrared spectrum. Transitioning between doped and undoped states alters various properties of Light-Emitting Polymers (LEPs), including polymer volume, absorption color, and reversible PL quenching. These controllable changes make LEPs highly promising for diverse applications: shifts in absorption color can be harnessed for electrochromic displays, while volume changes may be applied in electroactive artificial polymer muscles. The combination of semiconductivity and intense PL enables LEP electroluminescence, leading to their use in polymer light-emitting diodes (PLEDs). Additionally, the high sensitivity of PL quenching to doping or charge transfer allows for the detection of biological and explosive substances. Consequently, LEPs represent a significant class of low-temperature processable materials with broad scientific and technological potential.
PLEDs are currently being developed for flat-panel displays and lighting applications, with strong commercialization prospects contingent on a deeper understanding and enhancement of LEP properties. For instance, although PLEDs feature a relatively simple thin-film structure, as shown in Figure 1, high-performance PLEDs demand that the LEP layer meet several stringent criteria: (1) color purity, determined by the polymer bandgap and film morphology; (2) alignment of ionization potentials and electron affinities between the LEP and electrode materials; (3) high PL quantum efficiency; (4) chemical and thermal stability; and (5) processability, encompassing solubility, solution viscosity, and solvent-substrate compatibility. These properties can be fine-tuned by modifying the chemical structure of conjugated polymer chains, side groups, heteroatom incorporation, molecular weight, structural regularity, and copolymerization. This article reviews the primary categories of LEPs tailored for various applications. It is worth noting that LEPs encompass both polymers with fully conjugated main chains and those with conjugated segments in the main chain or side groups. Most conjugated oligomers exhibit similar processability and luminescent properties to their polymeric counterparts.
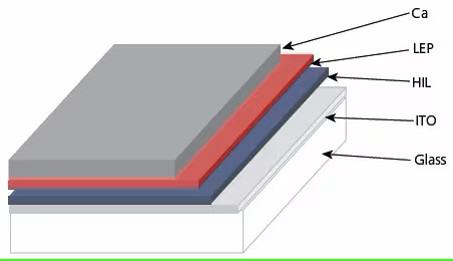
Figure 1. Schematic representation of a polymer light-emitting diode (PLED). HIL = hole injection layer, typically a spin-cast film of an inherently conductive polymer (PEDOT or polyaniline).
Poly(1,4-phenylene vinylene) (PPV)
References to polymer structures in Figure 2 are highlighted in bold. PPV (1) and its soluble derivatives are among the most extensively studied LEPs. PPV is generally synthesized via a precursor, poly(xylylidenetetrahydrothiophenium chloride), which is soluble in water and methanol. PPV was used in the earliest conjugated polymer light-emitting diodes (LEDs), but its relatively low PL quantum efficiency and high-temperature precursor conversion prompted the development of numerous new PPV derivatives soluble in common organic solvents. MEH-PPV can be conveniently synthesized from the monomer 1,4-bischloromethyl-2-methoxy-5-(2’-ethylhexyloxy) benzene (Scheme 1a). The dialkoxy side groups also modify the polymer’s bandgap, shifting the emission color bathochromically to orange from the yellow-green of unsubstituted PPV. MEH-PPV was utilized in the fabrication of the first high-efficiency polymer LEDs. The emission color shifts further toward red when the 2’-ethylhexyloxy side group is replaced by a 3’,7’-dimethyloctyloxy group (MDMO-PPV). In BCHA-PPV (3), where the side groups are bulky 2,5-bis(cholestanoxy), the color shifts to orange-yellow. Many other side groups have been employed to modify the emission color. Side groups can also be introduced to the vinyl moieties of PPV.
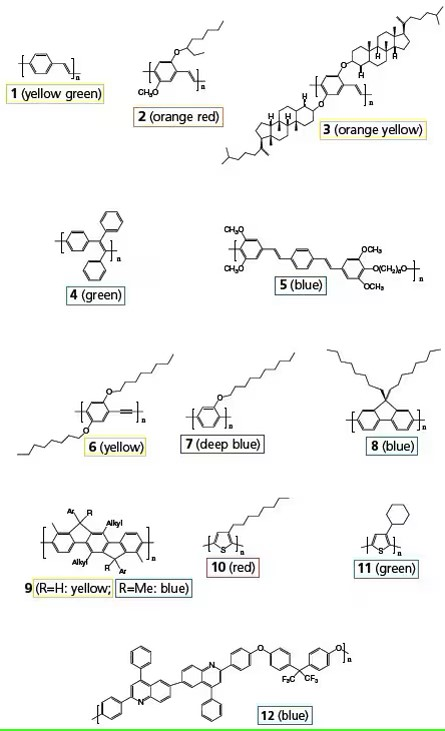
Figure 2. Representative classes of light-emitting polymers discussed in the following sections.
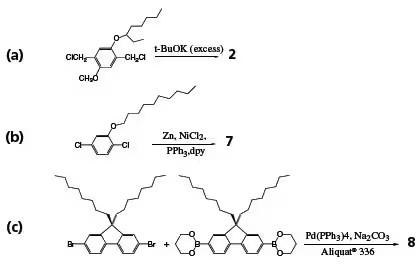
Scheme 1. Synthetic pathways to polymers (2), (7), and (8).
Gilch polymerization was employed to produce MEH-PPVs with high yield and molecular weight. In the Gilch reaction of 1,4-bischloromethyl-2,5-bis(3’,7’ -dimethyloctyloxy)benzene, 1.5–2.0% of tolane-bisbenzyl moieties were identified as the sole defects on the PPV main chain. In the polymerization of 1,4-bischloromethyl-2-methoxy-5-(3-decyloxyphenyl)benzene, the p-quinodimethane intermediate is highly polar. The subsequent free-radical polymerization predominantly yields head-to-tail coupling, resulting in a substituted PPV with 0.5% tolane-bisbenzyl defects. Such minimal defects are crucial for achieving high-performance polymer LEDs. Substituted PPVs with relatively low molecular weights can also be synthesized via condensation polymerization routes (Wittig, Heck, Knoevenagel). In Knoevenagel polymers, a cyano group is introduced to each vinylene moiety of dialkoxy-PPV, creating an intramolecular "push-pull" effect of π-electron clouds that effectively reduces the bandgap and shifts the emission color to the deep red region.
The emission color and the PL quantum efficiency of substituted PPV can be finessed via copolymers of PPV containing different side groups. For instance, varying the ratio of the comonomers in poly[(2-dimethyloctylsilyl-1,4-phenylene vinylene)-co-(MEH-PPV)], can systematically tune the emission color from green of the silyl-PPV to orange in MEH-PPV. To obtain emission colors in the green to blue range, one may adopt copolymers consisting of short segments of PPV strung together with non-conjugated moieties such as alkylenedioxy, oligo(ethylene oxide), and dimethylsilane. Copolymer (5) is an efficient blue emitter. These copolymers, however, generally exhibit poor charge carrier conductivity. Poly(2,5-dialkoxy-1,4- phenyleneethynylene) (PPE) are dehydro analogs of dialkoxy- PPV. The emission peak of the PPE (6) is narrower and blue shifted compared to corresponding PPVs.
Poly(1,4-phenylene) (PPP)
Blue LEPs can be derived from poly(1,4-phenylene) (PPP) and its derivatives. Soluble PPPs can be synthesized from dichloro, dibromo, or diborate monomers via Grignard, Ni0-catalyzed Yamamoto, or Suzuki reactions. However, the molecular weights of the resulting polymers are often low, typically below 10,000. High-molecular-weight soluble PPPs containing electron-withdrawing groups, such as benzoyl, can be obtained through Ni0-catalyzed Yamamoto coupling of corresponding dichloro monomers (Scheme 1b). All substituted PPPs emit deep blue light, with a significant portion of the emission in the UV region. Poly(2-decyloxy-1,4-phenylene) (7) exhibits high PL and EL quantum yields, with an emission peak around 410 nm.
Polyfluorenes (PFO)
Polyfluorene, where each pair of phenyl rings is locked in plane by the C-9 position, has a slightly smaller bandgap than PPP. Most of its PL falls within the blue region of the visible spectrum. In poly(9,9-dioctylfluorene) (8), the solubilizing alkyl groups are positioned on the C-9, far from the C-2 and C-7 positions, minimally interfering with the polymerization of corresponding monomers that link fluorene units through C-2 and C-7. Polymers with Mw > 100,000 and PL quantum yields > 70% have been achieved via Ni0-catalyzed Yamamoto coupling and Pd0-catalyzed Suzuki coupling polymerization. Using a surfactant (e.g., Aliquat® 336) to enhance solvent mixing in Suzuki polymerization (Scheme 1c) can further increase molecular weights up to 1,000,000. Alternating PF copolymers can be synthesized via the Suzuki route. Introducing triphenylamine comonomers enhances the hole-transporting ability of polyfluorenes, while thiophene and 1,3,4-benzothiadiazole comonomers reduce the bandgap, shifting the emission color toward green or red. Soluble derivatives of polyfluorenes and PPVs are currently commercialized for LED display and lighting products.
Further stabilization of the phenyl rings into a coplanar structure is achieved in ladder-type PPPs. Enhanced conjugation along the polymer backbone and excimer formation due to interchain interactions necessitate large side groups for solubility. The ladder polymer (9, R=H) exhibits intense blue luminescence in dilute solution. However, the PL of solid thin films shifts to yellow with only 10% quantum yield due to excimer formation. Replacing R with a methyl group suppresses excimer formation, resulting in solid thin films with intense blue luminescence, similar to the polymer in dilute solution.
Poly(thiophenes)
Poly(3-alkylthiophene)s have been widely studied for their thermo- and solvatochromism, as well as applications in field-effect transistors. Regiorandom poly(3-octylthiophene) (10) shows weak red PL in dilute solution, with emission largely quenched in concentrated solutions and solid thin films. Bulky side groups, such as cyclohexyl (11), disrupt the coplanarity of the polythiophene main chain, shifting the emission color to green. Poly(3-methyl-4-cyclohexylthiophene) emits blue light. Conversely, regioregular poly(3-alkylthiophene) features coplanar polymer main chains that can orderly pack into crystalline nanometer-sized domains with high hole mobility, making them suitable as p-type semiconductors for thin-film transistors and solar cells. Thiophene, 3-alkylthiophene, and 3-alkoxythiophene are frequently used as comonomers to reduce the bandgap of PPP, PF, and PPV.
Nitrogen-Containing Polymers
Heterocyclic rings containing imino-N act as electron acceptors when conjugated with hydrocarbon-based π-systems. Poly(2,5-pyridinevinylene) emits red light in dilute solutions, but its PL quantum efficiency is relatively low due to strong dipole interactions promoting aggregate formation. Protonation or alkylation of the N-atom causes complex changes in emission color and efficiency. Quinoline is a useful building block for PPP-type copolymers with high electron affinity. Polyquinoline (12) is an efficient blue emitter. 1,3,4-Oxadiazole, another heterocyclic aromatic ring, is often copolymerized with phenyl rings to increase electron affinity. Polymers and copolymers containing 1,3,4-oxadiazole with large bandgaps are commonly used as electron-transporting materials, while those with smaller bandgaps and visible light emission are employed in PLEDs. Conversely, tertiary amines and their derivatives are used as hole-transporting polymers. Poly(9-vinylcarbazole) (PVK) is a good photoconductive material and a popular wide-bandgap host for emissive materials like perylene and phosphorescent dopants.
Water-Soluble LEPs
Ionic groups such as quaternary ammonium and sulfonate can be attached to conjugated polymers via flexible tethers to render them soluble in water or methanol. Sulfonato-substituted polythiophene is self-dopable in water and shows negligible luminescence upon doping. Sulfonated PPV, PPP, and PF exhibit intense PL in dilute aqueous solutions, with emission colors similar to their non-sulfonated analogs. These water-soluble luminescent polymers have been studied for biosensing due to their high sensitivity to PL quenching by electron acceptors like methyl viologen.
Conclusion
Light-emitting polymers are characterized by (1) a high absorption coefficient, up to 105/cm; (2) high PL efficiency, with quantum efficiencies often exceeding 50% for blue and green emissive polymers in solid-state thin films; and (3) a large Stokes shift, minimizing self-absorption of PL emission. Polymer synthesis provides a versatile tool to tune these properties: Figure 3 illustrates LEPs covering the entire visible spectrum. LEPs enable a wide range of applications, including sensors, flexible LED displays, lighting devices, optical pump lasers, and potentially polymer diode lasers. However, the susceptibility of LEPs to environmental oxygen, moisture, and UV radiation may pose limitations and hinder the commercialization of some products.
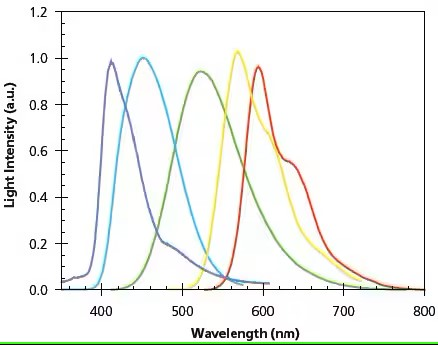
Figure 3. Film electroluminescence spectra of representative LEPs. Peak positions from left to right: (7), (12), (4), (3), (2).
References
1. Skotheim T, Elsenbaumer R, Reynolds J. 1997. Reynolds. Handbook of Conducting Polymers 2. Newyork: Marcel Dekker.
2. Akcelrud L. 2003. Electroluminescent polymers. Progress in Polymer Science. 28(6):875-962. https://doi.org/10.1016/s0079-6700(02)00140-5
3. McQuade DT, Pullen AE, Swager TM. 2000. Conjugated Polymer-Based Chemical Sensors. Chem. Rev.. 100(7):2537-2574. https://doi.org/10.1021/cr9801014
4. Argun AA, Aubert P, Thompson BC, Schwendeman I, Gaupp CL, Hwang J, Pinto NJ, Tanner DB, MacDiarmid AG, Reynolds JR. 2004. Multicolored Electrochromism in Polymers: Structures and Devices. Chem. Mater.. 16(23):4401-4412. https://doi.org/10.1021/cm049669l
5. Pei Q, Inganläs O. 1992. Conjugated polymers and the bending cantilever method: Electrical muscles and smart devices. Adv. Mater.. 4(4):277-278. https://doi.org/10.1002/adma.19920040406
6. Wudl F, Srdanov G. 1993. Conducting polymer formed of poly(2-methoxy,5-(2'-ethyl-hexyloxy)-p-phenylenevinylene). 5(189):136.
7. Kim ST, Hwang D, Li XC, Grüner J, Friend RH, Holmes AB, Shim HK. 1996. Efficient green electroluminescent diodes based on poly (2-dimethyloctylsilyl-1,4-phenylenevinylene). Adv. Mater.. 8(12):979-982. https://doi.org/10.1002/adma.19960081206
8. Spreitzer H, Becker H, Kluge E, Kreuder W, Demandt R, Schoo H. 1998. Adv. Mater.. 101340–1343.
9. Johansson DM, Srdanov G, Yu G, Theander M, Inganäs O, Andersson MR. 2000. Synthesis and Characterization of Highly Soluble Phenyl-Substituted Poly(p-phenylenevinylenes). Macromolecules. 33(7):2525-2529. https://doi.org/10.1021/ma991582b
10. Becker H, Spreitzer H, Ibrom K, Kreuder W. 1999. New Insights into the Microstructure of GILCH-Polymerized PPVs. Macromolecules. 32(15):4925-4932. https://doi.org/10.1021/ma990347q
11. Kraft A, Grimsdale A, Holmes A. 1998. Angew. Chem. Int.. 37402.
12. Hu B, Karasz FE. 2003. Blue, green, red, and white electroluminescence from multichromophore polymer blends. Journal of Applied Physics. 93(4):1995-2001. https://doi.org/10.1063/1.1536018
13. Montali A, Smith P, Weder C. 1998. Poly(p-phenylene ethynylene)-based light-emitting devices. Synthetic Metals. 97(2):123-126. https://doi.org/10.1016/s0379-6779(98)00120-9
14. Rehahn M, Schlüter A, Wegner G. 1990. Makromol. Chem.. 191(9):1991-2003. https://doi.org/10.1002/macp.1990.021910902
15. Yang Y, Pei Q, Heeger AJ. 1996. Efficient blue polymer light-emitting diodes from a series of soluble poly(paraphenylene)s. J. Appl. Phys.. 79(2):934. https://doi.org/10.1063/1.360875
16. Fukuda M, Sawada K, Yoshino K. 1993. Synthesis of fusible and soluble conducting polyfluorene derivatives and their characteristics. J. Polym. Sci. A Polym. Chem.. 31(10):2465-2471. https://doi.org/10.1002/pola.1993.080311006
17. Pei Q, Yang. 1996. Efficient Photoluminescence and Electroluminescence from a Soluble Polyfluorene. J. Am. Chem. Soc.. 118(31):7416-7417. https://doi.org/10.1021/ja9615233
18. Inbasekaran M, Woo E, Wu W, Bernius M, Wujkowski L. 2000. Fluorene homopolymers and copolymers. Synthetic Metals. 111-112397-401. https://doi.org/10.1016/s0379-6779(99)00382-3
19. Bernius M, Inbasekaran M, Woo E, Wu W, Wujkowski L. 2000. 11(2):111-116. https://doi.org/10.1023/a:1008917128880
20. Scherf U. 1999. Ladder-type materials. J. Mater. Chem.. 9(9):1853-1864. https://doi.org/10.1039/a900447e
21. Andersson MR, Berggren M, Inganaes O, Gustafsson G, Gustafsson-Carlberg JC, Selse D, Hjertberg T, Wennerstroem O. 1995. Electroluminescence from Substituted Poly(thiophenes): From Blue to Near-Infrared. Macromolecules. 28(22):7525-7529. https://doi.org/10.1021/ma00126a033
22. Wang YZ, Epstein AJ. 1999. Interface Control of Light-Emitting Devices Based on Pyridine-Containing Conjugated Polymers. Acc. Chem. Res.. 32(3):217-224. https://doi.org/10.1021/ar980052h
23. Pei Q, Yang Y. 1995. 1,3,4-Oxadiazole-Containing Polymers as Electron-Injection and Blue Electroluminescent Materials in Polymer Light-Emitting Diodes. Chem. Mater.. 7(8):1568-1575. https://doi.org/10.1021/cm00056a025
24. Huang F, Wu H, Wang D, Yang W, Cao Y. 2004. Novel Electroluminescent Conjugated Polyelectrolytes Based on Polyfluorene. Chem. Mater.. 16(4):708-716. https://doi.org/10.1021/cm034650o
25. Chen L, McBranch DW, Wang H, Helgeson R, Wudl F, Whitten DG. 1999. Highly sensitive biological and chemical sensors based on reversible fluorescence quenching in a conjugated polymer. Proceedings of the National Academy of Sciences. 96(22):12287-12292. https://doi.org/10.1073/pnas.96.22.12287
Aladdinsci: https://www.aladdinsci.com
