Lithium

Product Manager
Sandra Forbes
Name Reactions
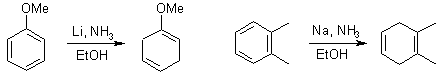
Birch Reduction
The Birch Reduction provides a means to obtain substituted 1,4-cyclohexadienes.
Recent Literature
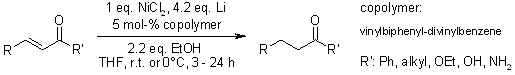
A system consisting of nickel(II) chloride, lithium metal, a catalytic polymer-bound arene, and ethanol has been effectively utilized for the conjugate reduction of diverse α,β-unsaturated carbonyl compounds under extremely gentle reaction conditions.
F. Alonso, I. Osante, M. Yus, Synlett, 2006, 3017-3020.
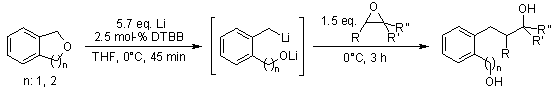
At 0°C, dianions resulting from the reductive ring-opening of phthalan (n=1) or isochroman (n=2) with lithium and a catalytic quantity of 4,4′-di-tert-butylbiphenyl (DTBB) underwent reactions with various epoxides, yielding 1,6-diols and 1,7-diols, respectively.
M. Yus, T. Soler, F. Foubelo, Tetrahedron, 2002, 58, 7009-7016.
DOI: 10.1016/S0040-4020(02)00696-8
The reductive detritylation of aliphatic and aromatic secondary and tertiary N-tritylamines, facilitated by lithium powder and a catalytic amount of naphthalene, efficiently produced the corresponding amines in good yields. Notably, the trityl group could be selectively removed even in the presence of an allyl or benzyl group.
C. Behloul, D. Guijarro, M. Yus, Synthesis, 2004, 1274-1280.
At low temperatures in THF, the reaction of various protected alcohols, amines, and amides with lithium and a catalytic quantity of naphthalene results in their deprotection under exceedingly gentle conditions. This process is frequently chemoselective.
E. Alonso, D. J. Ramón, M. Yus, Tetrahedron, 1997, 53, 14355-14368.
DOI: 10.1016/S0040-4020(97)00920-4
E. Alonso, D. J. Ramón, M. Yus, Tetrahedron, 1997, 53, 14355-14368.
DOI: 10.1016/S0040-4020(97)00920-4
E. Alonso, D. J. Ramón, M. Yus, Tetrahedron, 1997, 53, 14355-14368.
DOI: 10.1016/S0040-4020(97)00920-4
At room temperature in tetrahydrofuran, a reducing system comprising CuCl2·2H2O, an excess of lithium sand, and a catalytic amount of 4,4′-di-tert-butylbiphenyl (DTBB) effectively reduced a series of alkyl sulfonates to their corresponding hydrocarbons. This process was also applicable to enol and dienol triflates, yielding alkenes and dienes, respectively.
G. Radivoy, F. Alonso, Y. Moglie, C. Vitale, M. Yus, Tetrahedron, 2005, 61, 3859-3864.
DOI: 10.1016/j.tet.2005.01.078
Partial Reduction of Aromatic Compounds without Ammonia Utilizing Lithium Di-tert-butylbiphenyl (LiDBB)
T. J. Donohoe, D. House, J. Org. Chem., 2002, 67, 5015-5018.
DOI: 10.1021/jo0257593
2-Substituted pyrrolidines, including nornicotine, can be synthesized from basic materials like carbonyl compounds (primarily aromatic aldehydes) and 3-chloropropylamine. However, when imines are derived from aliphatic ketones and aldehydes, this method results in significantly reduced yields.
M. Yus, T. Soler, F. Foubelo, J. Org. Chem., 2001, 66, 6207-6208.
DOI: 10.1021/jo010419n
At room temperature, 2-lithioimidazole was synthesized using lithium metal in the presence of a catalytic quantity of isoprene in THF. When this organolithium compound was reacted with carbonyl electrophiles, it yielded 2-(hydroxyalkyl)imidazoles and 2-(aminoalkyl)imidazoles in good amounts.
R. Torregrosa, I. M. Pastor, M. Yus, Tetrahedron, 2005, 61, 11148-11155.
DOI: 10.1016/j.tet.2005.09.024
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/lithium.shtm
Aladdinsci: https://www.aladdinsci.com








