Lithium triethylborohydride, LiTEBH, Superhydride

Product Manager
Sandra Forbes
LiBHEt3 is an exceptionally potent reducing agent, surpassing even lithium aluminum hydride in efficiency. For instance, LiTEBH facilitates rapid reduction of carbonyl compounds, esters, acid chlorides, acid anhydrides, and tertiary amides into alcohols, as well as the conversion of disulfides into thiols.
Recent Literature
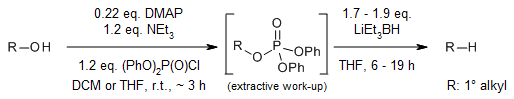
At room temperature, primary alcohols can be efficiently deoxygenated with high yields through the reduction of derived diphenyl phosphate esters using lithium triethylborohydride in THF. This process allows for the selective reduction of primary alcohols even in the presence of secondary alcohols. Furthermore, the adoption of a one-pot two-step procedure simplifies and facilitates the entire process.
S. Chowdhury, R. F. Standaert, J. Org. Chem., 2016, 81, 9957-9963.
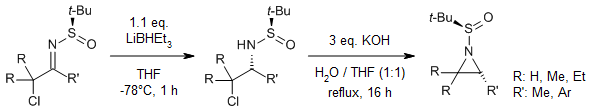
The reduction of (Rs)-N-tert-butanesulfinyl α-halo imines with NaBH4 in THF, in the presence of 10 equivalents of MeOH, followed by cyclization with KOH, resulted in the quantitative formation of the corresponding (Rs,S)-N-(tert-butylsulfinyl)aziridines. Conversely, by switching the reducing agent from NaBH4 to LiBHEt3, the epimer (Rs,R)-N-(tert-butylsulfinyl)aziridine was synthesized with good yields and diastereoselectivity.
B. Denolf, E. Leemans, N. De Kimpe, J. Org. Chem., 2007, 72, 3211-3217.
DOI: 10.1021/jo0624795
Quoted from:
https://www.organic-chemistry.org/chemicals/reductions/lithiumtriethylborohydride.shtm
Aladdinsci: https://www.aladdinsci.com
