Loading Controls for Western Blotting

Since the first publication describing Western blotting, this immunodetection technique has been widely used to identify specific proteins in complex mixtures extracted from cells or tissues. Western blotting has three basic elements:1 separation of proteins by size,2 transfer to a solid support and 3 labeling of target proteins with an appropriate primary antibody followed by visualization, typically with a conjugated secondary antibody. Subsequent refinements in tools and techniques along with the development of highly sensitive fluorescent labels have greatly improved the limits of detection, allowing scientists to study tissue-specific normal and disease pathways. However, scientists typically face some difficulties when using quantitative Western blotting to identify changes in protein levels. This is because many proteins have different expression patterns in different tissues and under different physiological and pathological conditions.
WESTERN BLOTTING: A ROUTINE TECHNIQUE THAT IS NOT SIMPLE
Whilst Western blotting has become the most prevalent immuno-application, a few crucial technical issues tend to escape attention. Specifically, do isolation or separation protocols impact on protein integrity or any post-translation modifications? Is it possible to distinguish between protein degradation or aggregation and relevant biological product? In cases where multiple bands result, how can one discern between results, variations or artifacts?
Quantitative western blot allows comparison of relative protein amounts in a sample group that may represent various individuals, treatment conditions, disease states, or other biological variables. Loading controls are employed as internal standards to identify and accurately measure total protein levels across multiple samples. This control involves adding a primary antibody against a protein assumed to be present in all samples and whose relative abundance is not affected by biological variation or experimental conditions. "Housekeeping" gene products, which are widely expressed protein targets, are generally suitable candidates for loading controls. The use of loading controls enables the sample loading to be quantified across all wells to normalize results with the assumption that the loading control is consistent between different sample lanes. Additionally, loading controls protect against the “edge effect” which is commonly observed when a large number of lanes are used. In this scenario, proteins in the outer lanes transfer to the membrane closer to the frame, causing more intense staining at the edge of the blot. Loading controls can demonstrate whether there has been any disparity in protein loading and whether this may explain any differences seen in the target band(s). Proper use of loading controls ensures that proteins are quantified accurately in spite of slight differences in loading amounts throughout all lanes of the Western blot.
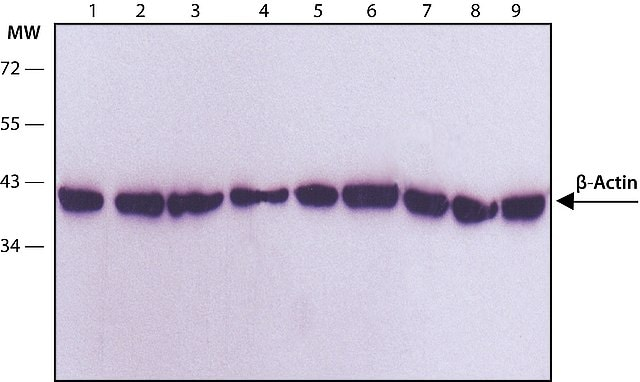
CHOOSING LOADING CONTROL ANTIBODIES
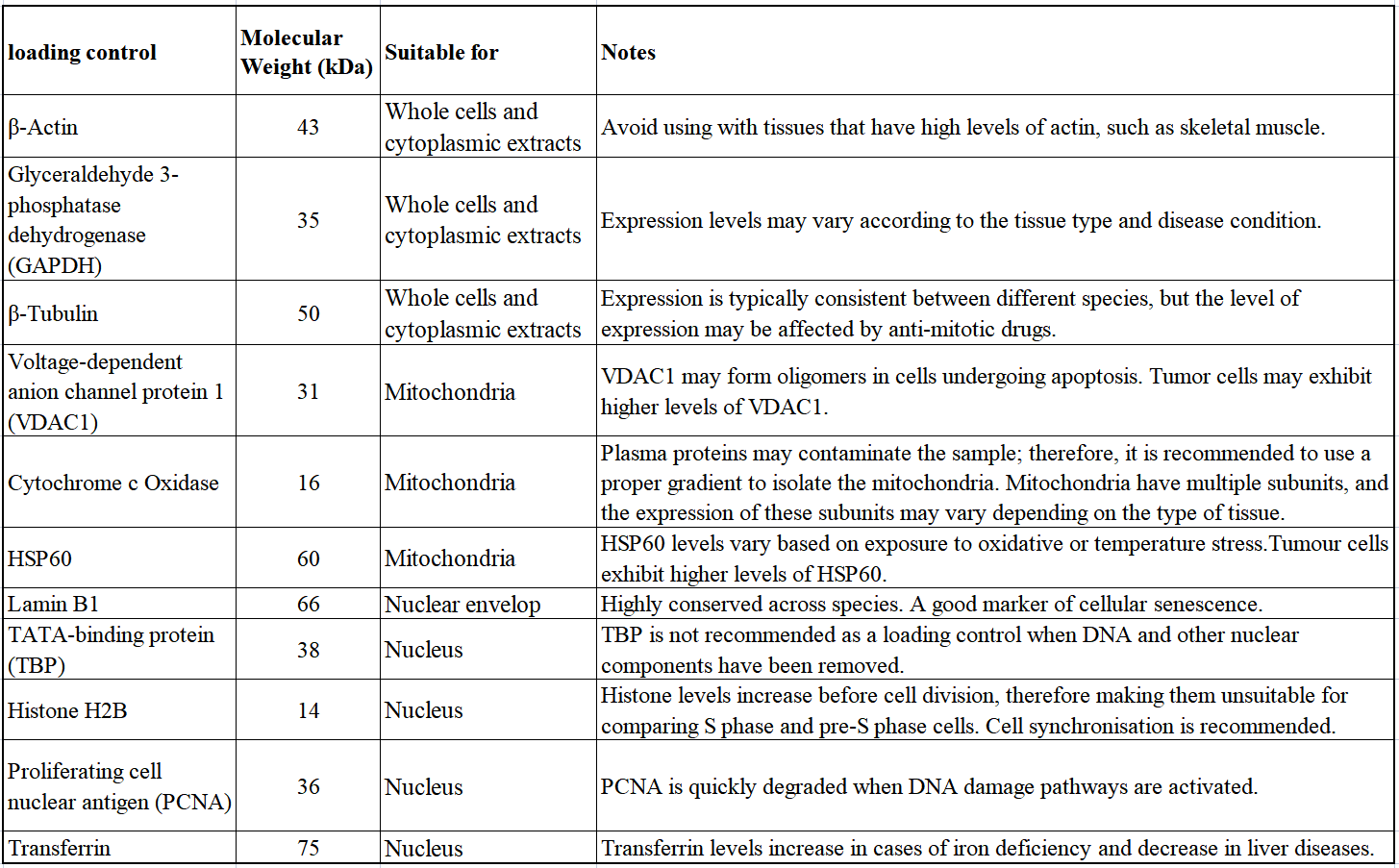
β-actin and β-tubulin are commonly used as loading controls due to their relatively consistent expression in most model systems. Nevertheless, several publications have raised concerns about the suitability of β-actin as a standard loading control, given that the levels of β-actin and β-tubulin can vary between tissues and pathological conditions can affect their expression. The authors suggest using a total protein analysis as an alternative technique in quantitative Western blotting. They recommend caution when using "housekeeping" gene products, and suggest studying the expression pattern of the gene under investigation beforehand. However, β-actin and β-tubulin are advantageous loading controls as they demonstrate high conservation, exhibit high expression levels, and remain stable under most experimental conditions. It is crucial to select an appropriate control depending on the studied tissue or cell type. Empirical testing may also be necessary to confirm the consistency of the loading control.
TIPS FOR GETTING EFFECTIVE RESULTS FROM LOADING CONTROL ANTIBODIES
To minimize variability in Western blotting and avoid errors in data interpretation, please follow these precautions.
Choose internal loading controls that are stably expressed and minimally affected by experimental conditions.
Select a loading control antibody that targets a protein known to be constitutively expressed in your sample.
Use a second loading control to confirm the results obtained from the first control, especially when dealing with new or novel samples.
Loading controls should cover a broad range of molecular weights, so that the chosen control is in a similar MW range, but not identical to the target protein. This guarantees that both target and control bands can be differentiated easily on the blot.
Loading control antibodies often detect "housekeeping" proteins that are highly expressed, which can lead to signal saturation, especially when using a chemiluminescent detection technique. Over-saturation can render loading control bands ineffective as a reference and obscure variations in target protein quantity between samples.
Titrate the concentration of the antibody and the exposure time of the loading control blot with the sample to be used beforehand to ensure that the signal of the loading control remains within the linear range of detection.
Reference
1. Renart J, Reiser J, Stark GR. 1979. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure.. Proceedings of the National Academy of Sciences. 76(7):3116-3120. https://doi.org/10.1073/pnas.76.7.3116
2. Dittmer A, Dittmer J. 2006. β-Actin is not a reliable loading control in Western blot analysis. Electrophoresis. 27(14):2844-2845. https://doi.org/10.1002/elps.200500785
3. Eaton SL, Roche SL, Llavero, Hurtado M, et al. Total Protein Analysis as a Reliable Loading Control for Quantitative Fluorescent Western Blotting. PLoS ONE. 8(8):e72457. https://doi.org/10.1371/journal.pone.0072457
4. Li R, Shen Y. 2013. An old method facing a new challenge: Re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sciences. 92(13):747-751. https://doi.org/10.1016/j.lfs.2013.02.014
5. Gorr TA, Vogel J. 2015. Western blotting revisited: Critical perusal of underappreciated technical issues. Prot. Clin. Appl.. 9(3-4):396-405. https://doi.org/10.1002/prca.201400118
