Procedures and precautions for the preparation of mouse peripheral blood single-cell suspensions
Preparation of mouse peripheral blood single cell suspension
1. Collect mouse peripheral blood samples in an anticoagulation tube. 2.
2. Add 100 μL of fresh blood into the centrifuge tube, add 1 Test corresponding to the flow antibody, mix well, and incubate at 4℃ for 30 min away from light.
3. Add 2 mL of 1× red blood cell lysate, mix well, and lysed at 4℃ for 5 min. 4.
4. Centrifuge at 300 g for 5 min (centrifuge immediately after lysis to prevent prolonged damage to the cells), and discard the supernatant to obtain white cell precipitate.
5. Wash with PBS.
6. Add 200 μL of cell staining buffer to resuspend the cells, and detect and analyze by flow cytometry.
FSS/SSC map of peripheral blood in C57 mice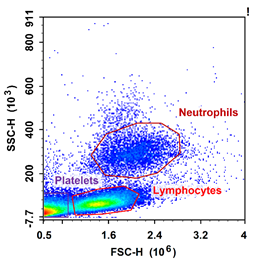

Precautions:
1. Anticoagulation tubes for blood collection, there are two kinds of heparin and EDTA, both kinds of anticoagulation tubes can be used if subsequent staining experiments are carried out after direct lysis of erythrocytes; heparin anticoagulation must be used if PBMC cell separation is carried out after blood collection.
2. E-CK-A105 10× ACK Lysis Buffer without fixative is recommended for lysis solution.
3. 10× ACK Lysis Buffer should be diluted to 1× with pure water before the experiment, ready for use, and recommended to be stored temporarily at 4℃ and used on the same day.
4. For the detection of routine indicators in mouse peripheral blood, overnight preserved samples can be used, which is generally not a big problem, but for indicators with low expression, it is recommended to use fresh samples for detection.
5. Mouse peripheral blood with low cell volume is recommended to be stained and then lysed. This method can reduce the number of washes during sample processing, thus reducing cell loss and avoiding some cells with a low percentage being undetectable.
For more information, visit our website: www.aladdinsci.com.
