N-Fluoro-2,4,6-trimethylpyridinium triflate
Product Manager:Nick Wilde


Recent Literature
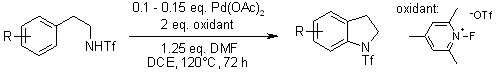
Pd(II)-catalyzed intramolecular amination of arenes using either Ce(SO4)2 as a one- or N-fluoro-2,4,6-trimethylpyridinium triflate as a two-electron oxidant tolerates a wide range of functional groups including acetyl, cyano, and nitro. This catalytic reaction allows expedient syntheses of broadly useful substituted indolines or indoles.
T.-S. Mei, X. Wang, J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 10806-10807.
https://doi.org/10.1021/ja904709b
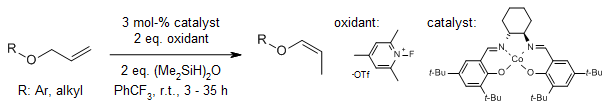
An efficient Z-selective oxidative isomerization process of allyl ethers catalyzed by a cobalt(II) (salen) complex using N-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (Me3NFP•OTf) as an oxidant provides thermodynamically less stable Z-enol ethers in excellent yields with high geometric control. Diallyl ethers can also be isomerized at room temperature.
G.Huang, M. Ke, Y. Tao, F. Chen, J. Org. Chem., 2020, 85, 5321-5329.
https://doi.org/10.1021/acs.joc.0c00004
Quoted from:https://www.organic-chemistry.org/chemicals/oxidations/n-fluoro-2,4,6-trimethylpyridinium-triflate.shtm
Aladdin:https://www.aladdinsci.com
