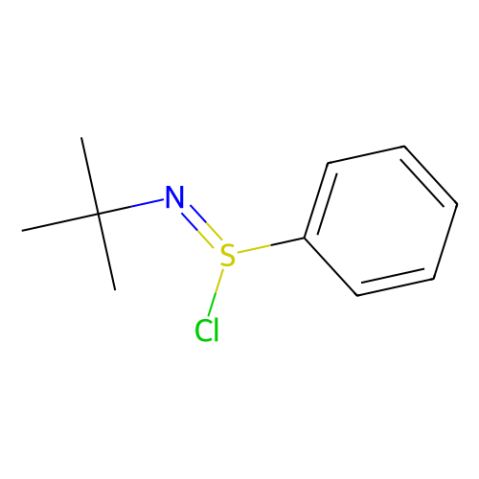
N-tert-butylbenzenesulfinimidoyl chloride
 _%E8%8B%B1%E6%96%872.jpg?access_token=3d151b21-353f-4c37-af8c-ab39c0dedc56)
Product Manager:Nick Wilde
Recent Literature

A range of unsymmetrical ketones has been prepared in good yields from aldehydes in one simple synthetic operation by addition of organolithium compounds followed by an oxidation using N-tert-butylphenylsulfinimidoyl chloride.
J. J. Crawford, K. W. Hederson, W. J. Kerr, Org. Lett., 2006, 8, 5073-5076.
https://doi.org/10.1021/ol061903l

A one-pot procedure enables the conversion of various ketones into α,β-unsaturated ketones in very good yields under mild conditions via treatment of lithium enolates with N-tert-butyl phenylsulfinimidoyl chloride.
T. Mukaiyama, J.-i. Matsuo, H. Kitagawa, Chem. Lett., 2000, 1250-1251.
https://doi.org/10.1246/cl.2000.1250
Efficient carbon-carbon bond formation of N-carbobenzyloxy amines with 1,3-dicarbonyl compounds at the α-position of nitrogen was established by a one-pot oxidative Mannich reaction using N-tert-butylbenzenesulfinimidoyl chloride as an oxidant.
J-I. Matsuo, Y. Tanaki, H. Ishibashi, Org. Lett., 2006, 8, 4371-4374.
https://doi.org/10.1021/ol0618095
Quoted
Aladdin:https://www.aladdinsci.com

