Oxidizing Agent-Benzaldehyde
Product Manager:Nick Wilde

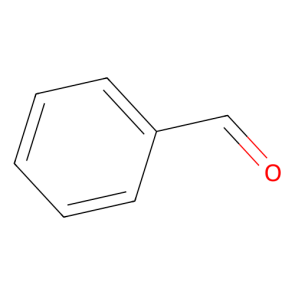
Benzaldehyde(C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor, and is commonly used in cherry-flavored sodas. A component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods.
Rcent Literature

Ruthenium-catalyzed oxidation of multisubstituted allyl alcohols in the presence of benzaldehyde gives enals or enones in good yields via an intramolecular hydrogen transfer. This reaction offers an efficient, mild, and high-yielding access to substituted α,β-unsaturated compounds.
K. Ren, B. Hu, M. Zhao, Y. Tu, X. Xie, Z. Zhang, J. Org. Chem., 2014, 79, 2170-2177. https://doi.org/10.1021/jo500042h
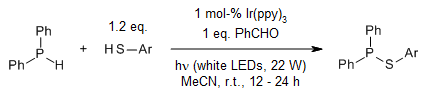
Visible-light photoredox catalysis achieved a dehydrogenative coupling of phosphines and thiophenols at room temperature. Key to this success is the use of benzaldehyde as a soft oxidant, which avoids phosphine oxidation. Furthermore, an unexpected dealkylative coupling of secondary and tertiary alkylphosphines with thiophenols is observed.
X. Wang, C. Xia, L. Wu, Org. Lett., 2020, 22, 7373-7377.
https://doi.org/10.1021/acs.orglett.0c02746
Quoted from: https://en.wikipedia.org/wiki/Benzaldehyde
https://www.organic-chemistry.org/chemicals/oxidations/benzaldehyde.shtm
Aladdin:https://www.aladdinsci.com
