Poly(Glycerol Sebacate) in Tissue Engineering
Sandra Forbes
Product Manager

Introduction
Over the past three decades, the realm of commercial biomaterials has encountered a lull, with scarce advancements transitioning from laboratory bench to clinical applications. Synthetic aliphatic polyesters have long maintained their stronghold in the realm of resorbable biomaterials, credited to their established track record and FDA approval. Despite extensive research endeavors aimed at crafting biocompatible and biodegradable polymers, emerging biomaterials have grappled with compliance mismatches, struggling to replicate the mechanical intricacies of natural tissues.
In response to these challenges, Professor Robert Langer's laboratory introduced poly(glycerol sebacate) (PGS), a resilient, biodegradable elastomer. Since its inception, PGS has garnered widespread adoption within the biomedical engineering community, finding applications in various implantable systems, spanning cardiovascular, neurovascular, orthopedic, and soft tissue domains.
PGS, a straightforward glycerol-ester polymer, is derived from fundamental mammalian metabolites—glycerol and sebacic acid—both of which enjoy an established regulatory standing with the FDA. Initially conceived as a biodegradable polymer boasting enhanced elastic properties and biocompatibility, subsequent research into PGS-based medical uses has unveiled a suite of exceptional characteristics, elevating its status as a versatile biomaterial. Beyond its elasticity, PGS exhibits minimal swelling, undergoes surface degradation, and elicits mild inflammatory responses, both acute and chronic, in living organisms.
While the thermoset elastomer form of PGS remains the preferred choice among researchers, the polymer's versatility allows for customization across a spectrum of resin forms. By adjusting the degree of polymerization, PGS can be tailored to suit diverse applications, ranging from soft gels and Vaseline®-like pastes to thermoplastics and thermosets. This morphological flexibility further enables PGS to be formulated as coatings for various medical implants, extruded into intricate luminal structures, sheets, rods, and other geometric configurations.
Moreover, PGS's compatibility with an array of biological materials, including collagen, bone minerals, and extracellular matrix (ECM)-mimicking compositions, underscores its ideality as a bioresorbable material for tissue engineering, regenerative medicine endeavors, and the broader biomedical device industry.
Poly(Glycerol Sebacate)
Design and Structure
The growing fascination with bioelastomers in tissue engineering primarily stems from the pressing requirement for soft-tissue repair solutions. PGS, meticulously crafted for the engineering of soft tissues exposed to dynamic mechanical conditions, like those within the cardiovascular system, is a testament to this pursuit. Crafted through polycondensation of glycerol and sebacic acid, PGS features ester bonds in its polymeric backbone that undergo covalent crosslinking, weaving a three-dimensional (3D) matrix of random coils. This intricate architecture echoes the structure of vulcanized rubber, conferring upon PGS a rubber-like elasticity, as depicted in Figure 1.
Additionally, hydrogen bonding interactions between hydroxyl groups found within PGS serve to fortify its mechanical prowess. The very nature of its ester linkages facilitates both the backbone and crosslinks of the polymer to undergo hydrolytic degradation, underscoring its biodegradability.

Figure 1. The synthesis of poly(glycerol sebacate) (PGS) involves a reaction scheme where glycerol and sebacic acid are combined as precursors. This mixture is then subjected to a curing process under heat and vacuum conditions, leading to the formation of a crosslinked PGS thermoset. This thermoset exhibits enhanced structural integrity and stability, thanks to the establishment of covalent crosslinks between the polymer chains.
Synthesis and Mechanical Properties
The synthesis of PGS commences with a polycondensation reaction between glycerol and sebacic acid, yielding a pre-polymer resin. This resin is subsequently transformed into a thermoset elastomer through a curing process. Notably, both starting materials are cost-effective and sourced from renewable resources, with sebacic acid being derived from castor oil. The synthesis process is environmentally benign, eschewing the use of toxic solvents or catalysts, contributing to an overall sustainable production of this biomaterial.
Among the various synthesis routes documented, the two-step method is prevalent. The initial step involves charging the reactor with the monomers and heating them to 120 °C under a nitrogen atmosphere to form a homogeneous solution. This mixture is then heated for 24 hours before being subjected to vacuum (ranging from 40 mTorr to 10 Torr) for an additional 24 to 48 hours, depending on the desired degree of polymerization. The resulting resin can be utilized in various forms, including as a casted film, molded into specific shapes, or dissolved in solvents for casting or dip-coating applications.
The resin is then cured for 24 to 96 hours to obtain the thermoset elastomer, with the curing time influencing the desired mechanical properties. One of PGS's strengths lies in its tunable mechanical properties, achieved by adjusting the polymerization and curing conditions. The modulus of the elastomer can be varied from 0.77 to 1.9 MPa by altering the cure time, while manipulating the monomer stoichiometry enables a broader range of modulus values (0.01–5 MPa), along with fine-tuning of molecular weight and chemical functionality. The average molecular weight can be adjusted from 2,000 to over 200,000 Da by changing the glycerol-to-sebacic acid ratio, while the chemical functionality, measured by acid number titration, ranges from 110 to 10 mg/g, impacting the elastomer's hydrophilicity and reactivity.
Degradability and Biocompatibility
PGS undergoes degradation primarily via hydrolysis of its ester bonds, resulting in the breakdown into smaller oligomers and eventually reverting to its original monomers: glycerol and sebacic acid. This degradation mechanism sets PGS apart from other resorbable polymers, as it proceeds through surface erosion rather than bulk erosion. This unique characteristic ensures a linear and controlled decline in mechanical properties over time, in contrast to the abrupt loss observed in bulk erosion.
While in vitro degradation studies have been conducted to predict in vivo performance, there is a notable discrepancy between the two, with in vitro models typically showing a more modest mass loss compared to the accelerated degradation observed in vivo. Nevertheless, the degradation rate of PGS can be tailored by adjusting the crosslinking density during the curing process, which is influenced by factors such as cure time and temperature.
The breakdown of PGS into its constituent monomers, glycerol and sebacic acid, underscores its high biocompatibility. Glycerol, a fundamental component in lipid metabolism, has a well-established safety profile in pharmaceutical applications. Sebacic acid, meanwhile, serves as an intermediate in the ω-oxidation of fatty acids and is incorporated into copolymers used for chemotherapeutic drug delivery.
Extensive research has evaluated the biocompatibility of PGS, both in vitro and in vivo. In vitro assays have confirmed its non-cytotoxic nature, while in vivo implantation studies have demonstrated minimal inflammatory response and limited fibrous capsule formation, likely attributed to its surface degradation pattern.
Applications in Tissue Engineering and Regenerative Medicine
Over the past three decades, tissue engineering has witnessed significant advancements, fueled partly by the emergence of innovative biodegradable elastomeric biomaterials. The inherent elasticity present in all bodily tissues underscores the criticality of matching mechanical properties between native tissues and engineered constructs, as mismatches are often cited as a cause for implant/graft failure. When it comes to vascular tissue interfaces, the elasticity of the substrate and the mechanical stimuli it provides play pivotal roles in modulating cell functions and directing tissue development. Consequently, elastomeric materials have garnered recognition as a vital category of scaffold materials, particularly for applications aimed at regenerating vascular and other soft tissues.
Applications in Cardiovascular Tissue
The selection of materials for cardiovascular applications heavily relies on their mechanical properties, with PGS standing out due to its minimal plastic deformation, making it an appealing choice for engineering cardiovascular tissues. Addressing the challenge of creating small-diameter arterial grafts, highly porous PGS scaffolds have proven particularly effective in the construction of small arteries. Notably, PGS supports robust adhesion and proliferation of endothelial progenitor cells and smooth muscle cells (SMCs), with SMCs cultured in PGS scaffolds co-expressing elastin and collagen, resulting in highly compliant engineered blood vessels. Furthermore, while tropoelastin production remains consistent on both PGS and PLGA scaffolds, the elastic nature of PGS enables tropoelastin crosslinking into desmosine-crosslinked elastin. In a rat abdominal aorta model, PGS tubes reinforced with a polycaprolactone nanofiber sheath demonstrated successful remodeling into neoarteries within 3 months, closely mimicking the native artery in mechanical, biochemical, and anatomical aspects. Remarkably, these neoarteries integrated seamlessly with the host vasculature, pulsing synchronously with host arteries. After one year, the neoarteries contained elastin levels comparable to native arteries and exhibited regeneration in their adventitia. (Figure 2)
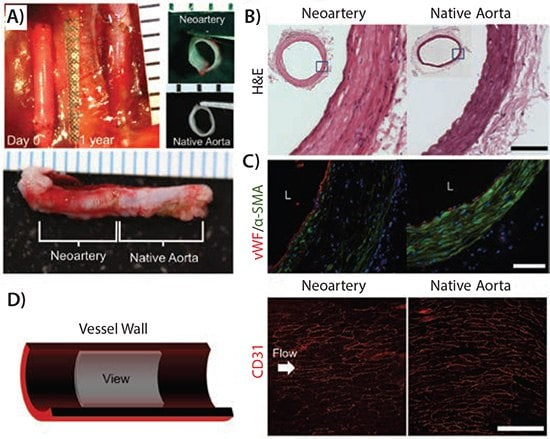
Figure 2. The neoarteries exhibit a gross morphology and tissue architecture that closely mimic those of native arteries. A) Over the course of a year, the graft undergoes a transformation into a neoartery in situ, as indicated by nondegradable sutures marking the graft's original location. Transverse and longitudinal views of explanted neoarteries reveal a striking resemblance to native aortas. B) Histological analysis of H&E-stained transverse sections from the middle of neoarteries shows a tissue architecture similar to native aortas, with no visible remnants of graft material. C) Immunostaining for von Willebrand factor (vWF) and α-smooth muscle actin (α-SMA) reveals a confluent endothelium covering the luminal surface of neoarteries, and a media-like middle layer rich in circumferentially elongated α-SMA-positive cells, akin to vascular smooth muscle in native aortas. The outermost layer lacks α-SMA, mirroring native adventitia. D) An en face view of the luminal surface of neoarteries, imaged using confocal microscopy, shows complete coverage by CD31-positive cells with a cobblestone-like morphology and alignment parallel to blood flow, similar to the arrangement found in native aortas. These findings demonstrate the successful remodeling and integration of neoarteries into the host vasculature. Reprinted with permission from Reference 13. Copyright 2013, Elsevier Ltd.
Polyglycolic acid (PGS) has garnered significant attention in cardiac tissue engineering owing to its versatility in tailoring mechanical properties to closely resemble those of myocardial tissues. This attribute has facilitated its utilization in various applications. For instance, PGS has been employed to craft highly porous scaffolds featuring parallel channels, which mimic the intricate capillary networks naturally present in the myocardium. By co-culturing cardiac fibroblasts and cardiomyocytes within a perfusion bioreactor augmented with oxygen carriers, functional contractile tissues were successfully generated within just 11 days.
Furthermore, when these cell-free PGS scaffolds were implanted into an infarcted rat myocardium model, they demonstrated remarkable vascularization within two weeks of implantation. Recent advancements include the development of a PGS scaffold with an innovative accordion-like honeycomb microstructure (depicted in Figure 3), where stiffness is precisely controlled through adjustment of the curing time to mirror the mechanical characteristics of rat right ventricular myocardium.
Moreover, PGS scaffolds have been pre-coated with extracellular matrix (ECM) proteins, serving as ligands to foster enhanced cell interactions. This strategy has been shown to promote increased cellularity, elevate ECM protein production, and modulate the differentiation of endothelial progenitor cells, further advancing the potential of PGS in cardiac tissue engineering.
Applications in Nerve Tissue
PGS has emerged as a promising scaffold material for nerve regeneration, with its neural biocompatibility thoroughly evaluated both in vitro and in vivo. In vitro experiments revealed that primary Schwann cells displayed comparable attachment rates and metabolic activities on both PGS and PLGA surfaces, but PGS surpassed PLGA in promoting higher cell proliferation and lower apoptosis. When implanted adjacent to the sciatic nerve in vivo, PGS elicited a significantly reduced chronic inflammatory response compared to PLGA, attributed to its minimal swelling and surface eroding properties.
A recent advancement saw the exploration of microfabricated PGS porous scaffolds for retinal progenitor cell (RPC) grafting. These scaffolds boasted mechanical properties (Young’s modulus of 1.66 ± 0.23 MPa and maximal strain of 113 ± 22%) that more closely mirrored those of retinal tissue than the conventional PLA/PLGA blend traditionally used for RPC delivery. In vitro studies demonstrated that RPCs adhered to and proliferated robustly within the PGS scaffold, exhibiting a promising trend towards differentiation. Furthermore, subretinal transplantations showed sustained RPC survival and robust migration into the host retinal tissue, underscoring the potential of PGS as a superior scaffold material for retinal tissue engineering.

Figure 3. Accordion-inspired honeycomb scaffolds exhibit anisotropic mechanical characteristics akin to those of native myocardial tissue. Panels A and B depict schematic illustrations of the accordion-like design, crafted by overlapping two 200 × 200 μm squares rotated 45° to form diamond-shaped units. These units align with the preferred (PD) and orthogonal cross-preferred (XD) directions, mirroring the circumferential and longitudinal axes of the heart, respectively. Scale bars indicate 1 mm for A and 200 μm for B.
Panel C showcases scanning electron micrographs, revealing the precision of excimer laser microablation in crafting the accordion-like honeycomb pattern in PGS. The scale bars measure 200 μm.
In Panel D, a systematic variation in PGS curing time is observed to linearly correlate with the effective stiffness (EPGS) of the material within the tested range.
Panel E presents representative uniaxial stress-strain curves for accordion-like honeycomb scaffolds seeded with neonatal rat heart cells. These scaffolds were fabricated from PGS membranes cured for 7.5 hours at 160°C and cultured with the heart cells for one week. This figure is reprinted with permission from Reference 19, copyright 2008, Nature Publishing Group.
Applications in Bone Tissue
Despite its rigidity, bone originates from pliable collagenous tissue during embryonic development. Likewise, the natural bone repair process commences with the emergence of a soft, temporary tissue known as callus. Given this insight, PGS elastomer scaffolds were employed to address non-union bone defects. Specifically, a porous PGS tube was implanted in a rabbit model to bridge the severed ends of an ulna. Within this experimental setup, healing initiated through the development of a cartilage-like tissue reminiscent of callus, which subsequently underwent mineralization, ultimately spanning the entire defect area within two months, as confirmed by micro-CT imaging.
The findings underscored that the relatively flexible PGS elastomer fostered an environment conducive to load transfer, thereby enhancing bone regeneration. Conversely, metallic implants were found to potentially impede healing by creating stress shielding effects on the bone.
Coating Applications for Medical Textiles
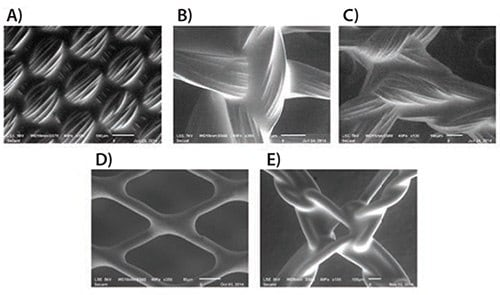
Coating technology holds a pivotal position in advancing medical device design by enabling the customization of substrates and augmentation of device performance. PGS has emerged as a highly promising coating material, exhibiting remarkable solubility in a diverse array of solvents (including ethyl acetate, THF, acetone, 1,3-dioxolane, and various alcohols), facilitating its utilization in dip and spray coating methods. Figure 4 showcases the versatility of PGS coating, as it seamlessly adheres to various textile substrates such as PET, polypropylene, PGA, and nitinol, forming a smooth, conformable thin film. This PGS coating imparts enhanced mechanical properties, elevated biocompatibility, and antimicrobial characteristics, underscoring its invaluable potential in the realm of medical device development.
Figure 4. Scanning Electron Microscope (SEM) imagery captures the intricate details of Regenerez coatings applied to a diverse spectrum of medical device textile components: A) poly(ethylene terephthalate) woven fabric subjected to dip-coating, B) poly(glycolic acid) knit material dip-coated, C) PEEK mesh treated with dip-coating, D) nitinol braid featuring spray-coating application, and E) poly(propylene) mesh undergone dip-coating. These insightful SEM images are courtesy of Carissa Smoot from the Secant Group.
Conclusions
Biomaterials will persist as cornerstone components in medical device technology and regenerative medicine, fueled by the perpetual need for materials that emulate the characteristics of native tissues. PGS, endowed with a comprehensive array of properties, stands as an exemplary material tailored to meet the multifaceted requirements of device and tissue engineering endeavors. Throughout the past decade and a half, PGS has garnered widespread adoption across cardiovascular, nervous, soft, and hard tissue applications, while continually unveiling novel avenues, including as coatings for implantable devices. This transition from research laboratories to commercialization, marked by the introduction of Regenerez® Poly(glycerol Sebacate) Resin, underscores PGS's ascendancy. The latest research breakthroughs, detailed herein, portend an even broader scope of applications and enhanced functionality for this versatile biomaterial.
References
1. Wang Y, Ameer GA, Sheppard BJ, Langer R. 2002. A tough biodegradable elastomer. Nat Biotechnol. 20(6):602-606. https://doi.org/10.1038/nbt0602-602
2. Rai R, Tallawi M, Grigore A, Boccaccini AR. 2012. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Progress in Polymer Science. 37(8):1051-1078. https://doi.org/10.1016/j.progpolymsci.2012.02.001
3. Chen Q, Liang S, Thouas GA. 2013. Elastomeric biomaterials for tissue engineering. Progress in Polymer Science. 38(3-4):584-671. https://doi.org/10.1016/j.progpolymsci.2012.05.003
4. Pomerantseva I, Krebs N, Hart A, Neville CM, Huang AY, Sundback CA. 2009. Degradation behavior of poly(glycerol sebacate). J. Biomed. Mater. Res.. 91A(4):1038-1047. https://doi.org/10.1002/jbm.a.32327
5. Wang Y, Kim YM, Langer R. 2003. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res.. 66A(1):192-197. https://doi.org/10.1002/jbm.a.10534
6. Chen Q, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. 2008. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 29(1):47-57. https://doi.org/10.1016/j.biomaterials.2007.09.010
7. Grego A, Mingrone G. 1995. Dicarboxylic acids, an alternate fuel substrate in parenteral nutrition: an update. Clinical Nutrition. 14(3):143-148. https://doi.org/10.1016/s0261-5614(95)80011-5
8. Liu G, Hinch B, Beavis AD. 1996. Mechanisms for the Transport of α,ω
-Dicarboxylates through the Mitochondrial Inner Membrane. J. Biol. Chem.. 271(41):25338-25344. https://doi.org/10.1074/jbc.271.41.25338
9. SUNDBACK C, SHYU J, WANG Y, FAQUIN W, LANGER R, VACANTI J, HADLOCK T. 2005. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 26(27):5454-5464. https://doi.org/10.1016/j.biomaterials.2005.02.004
10. Langer R, Vacanti J. 1993. Tissue engineering. Science. 260(5110):920-926. https://doi.org/10.1126/science.8493529
11. Freed LE, Engelmayr GC, Borenstein JT, Moutos FT, Guilak F. 2009. Advanced Material Strategies for Tissue Engineering Scaffolds. Adv. Mater.. 21(32-33):3410-3418. https://doi.org/10.1002/adma.200900303
12. GHOSH K, INGBER D. 2007. Micromechanical control of cell and tissue development: Implications for tissue engineering. Advanced Drug Delivery Reviews. 59(13):1306-1318. https://doi.org/10.1016/j.addr.2007.08.014
13. Crapo PM, Gao J, Wang Y. 2008. Seamless tubular poly(glycerol sebacate) scaffolds: High-yield fabrication and potential applications. J. Biomed. Mater. Res.. 86A(2):354-363. https://doi.org/10.1002/jbm.a.31598
14. Crapo PM, Wang Y. 2010. Physiologic compliance in engineered small-diameter arterial constructs based on an elastomeric substrate. Biomaterials. 31(7):1626-1635. https://doi.org/10.1016/j.biomaterials.2009.11.035
15. Gao J, Crapo P, Nerem R, Wang Y. 2008. Co-expression of elastin and collagen leads to highly compliant engineered blood vessels. J. Biomed. Mater. Res.. 85A(4):1120-1128. https://doi.org/10.1002/jbm.a.32028
16. Gao J, Ensley AE, Nerem RM, Wang Y. 2007. Poly(glycerol sebacate) supports the proliferation and phenotypic protein expression of primary baboon vascular cells. J. Biomed. Mater. Res.. 83A(4):1070-1075. https://doi.org/10.1002/jbm.a.31434
17. Wu W, Allen RA, Wang Y. 2012. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med. 18(7):1148-1153. https://doi.org/10.1038/nm.2821
18. Allen RA, Wu W, Yao M, Dutta D, Duan X, Bachman TN, Champion HC, Stolz DB, Robertson AM, Kim K, et al. 2014. Nerve regeneration and elastin formation within poly(glycerol sebacate)-based synthetic arterial grafts one-year post-implantation in a rat model. Biomaterials. 35(1):165-173. https://doi.org/10.1016/j.biomaterials.2013.09.081
19. Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. 2008. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nature Mater. 7(12):1003-1010. https://doi.org/10.1038/nmat2316
20. Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G. 2008. Cardiac tissue engineering using perfusion bioreactor systems. Nat Protoc. 3(4):719-738. https://doi.org/10.1038/nprot.2008.40
21. Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. 2006. Biomimetic Approach to Cardiac Tissue Engineering: Oxygen Carriers and Channeled Scaffolds. Tissue Engineering. 12(8):2077-2091. https://doi.org/10.1089/ten.2006.12.2077
22. Radisic M, Park H, Martens TP, Salazar-Lazaro JE, Geng W, Wang Y, Langer R, Freed LE, Vunjak-Novakovic G. 2008. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J. Biomed. Mater. Res.. 86A(3):713-724. https://doi.org/10.1002/jbm.a.31578
23. Sales VL, Engelmayr GC, Johnson JA, Gao J, Wang Y, Sacks MS, Mayer JE. 2007. Protein Precoating of Elastomeric Tissue-Engineering Scaffolds Increased Cellularity, Enhanced Extracellular Matrix Protein Production, and Differentially Regulated the Phenotypes of Circulating Endothelial Progenitor Cells. Circulation. 116(11_suppl):I-55-I-63. https://doi.org/10.1161/circulationaha.106.6806637
24. Neeley WL, Redenti S, Klassen H, Tao S, Desai T, Young MJ, Langer R. 2008. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials. 29(4):418-426. https://doi.org/10.1016/j.biomaterials.2007.10.007
25. Redenti S, Neeley WL, Rompani S, Saigal S, Yang J, Klassen H, Langer R, Young MJ. 2009. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 30(20):3405-3414. https://doi.org/10.1016/j.biomaterials.2009.02.046
26. Zaky SH, Lee K, Gao J, Jensen A, Close J, Wang Y, Almarza AJ, Sfeir C. 2014. Poly(Glycerol Sebacate) Elastomer: A Novel Material for Mechanically Loaded Bone Regeneration. Tissue Engineering Part A. 20(1-2):45-53. https://doi.org/10.1089/ten.tea.2013.0172
27. Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. 2008. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nature Mater. 7(12):1003-1010. https://doi.org/10.1038/nmat2316
For more information, visit our website:
Aladdinsci: https://www.aladdinsci.com/