Potential Applications in Click Chemistry
Click Chemistry serves as a potent technique for investigating the intracellular distribution of small molecules. Unveiling the precise cellular destinations of these compounds provides profound insights into their mechanisms of action.[1] This methodology has been extensively employed in various investigations, revealing significant findings such as the lysosomal localization of salinomycin, instigating ferroptosis in cancer stem cells[2], and the accumulation of metformin derivatives within mitochondria for copper(II) chelation, thereby influencing downstream metabolic and epigenetic alterations in inflammatory macrophages.[3]
The commercial viability of click chemistry is substantial. For instance, the conjugation of the fluorophore rhodamine onto norbornene, followed by reaction with tetrazine, has been successfully demonstrated in live systems.[4] Additionally, in different scenarios, strain-promoted azide-alkyne cycloaddition (SPAAC) between a cyclooctyne-modified fluorophore and azide-labeled proteins has facilitated the identification and selection of these proteins within cell lysates.[5]
The expansion of methodologies for integrating click reaction counterparts into systems both in vivo and ex vivo enhances the repertoire of feasible reactions. Advances in incorporating unnatural amino acids through ribosomal synthesis have facilitated the introduction of click reaction partners as unique side chains on these non-natural amino acids. For instance, the incorporation of an unnatural amino acid featuring an azide side chain allows for facile interaction with cycloalkynes in proteins tagged with this "AHA" unnatural amino acid.[6] In another illustration, "CpK" with a side chain containing a cyclopropane adjacent to an amide bond serves as a reactive partner for tetrazine in an inverse Diels-Alder reaction.[7]

The synthesis of luciferin illustrates an alternative approach to isolating reaction partners by exploiting naturally occurring, infrequent groups such as the 1,2-aminothiol. This particular group emerges exclusively when cysteine serves as the terminal N' amino acid in a protein. The inherent selectivity and bioorthogonality of these groups prove valuable in designing probes specific to such tags. The reaction involves the interaction between a 1,2-aminothiol and a 2-cyanobenzothiazole, resulting in the formation of fluorescent luciferin. Subsequent quantification of luciferin fluorescence through spectrometry, after a thorough wash, allows for the determination of the relative presence of the molecule bearing the 1,2-aminothiol. In cases where quantifying non-1,2-aminothiol-bearing proteins is necessary, the protein of interest can be enzymatically cleaved to produce a fragment with an N' Cys susceptible to the 2-CBT reaction.[8]
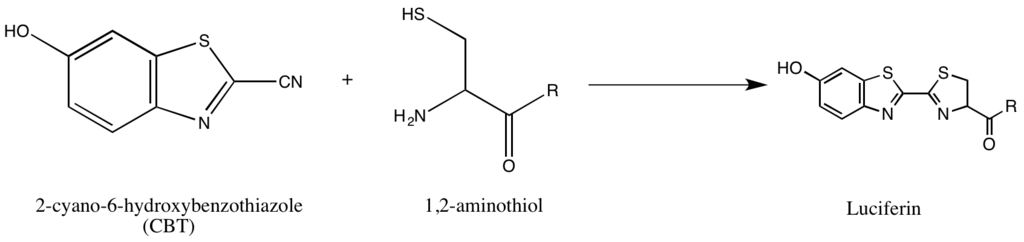
Scheme of the synthesis of firefly luciferin
The selective labeling of nascent proteomes by click chemistry, combined with two-dimensional difference gel electrophoresis (2D-DIGE), can be used to study the dynamic response of cells to changes in conditions. For example, incubation of cells with the methionine analogue hyperkynylglycine enables selective labeling of the nascent proteome with monosulfonated neutral Cy3 and Cy5 azides. Subsequent 2D DIGE isolation of fluorescently labeled proteins can then reveal the complexity of the nascent proteome after erythromycin treatment and shed light on the cellular response to various physiological conditions.[9]
In the field of polymers and biopolymers, vegetable oil-derived dienynes and diazides provide new synthetic routes for the fabrication of thermoplastic polytriazole polymers without solvents or catalysts via CuCCA click reactions. Since thermoplastic polytriazoles are novel polymers derived from vegetable oils, they have good mechanical and thermal properties and are suitable for a wide range of applications where residual solvents or catalysts cannot be tolerated. They are potentially biocompatible and biodegradable, making them ideal for applications in the medical and pharmaceutical industries.[10]
Teaming up with combinatorial chemistry, high-throughput screening, and the creation of chemical libraries, click chemistry has accelerated the pace of novel drug discovery. Its impact lies in streamlining each step of a multistep synthesis, rendering the process rapid, efficient, and predictable.
Reference
1. Cañeque, Tatiana; Müller, Sebastian; Rodriguez, Raphaël (2018). "Visualizing biologically active small molecules in cells using click chemistry". Nature Reviews Chemistry. 2 (9): 202–215. https://doi.org/10.1038/s41570-018-0030-x
2. Mai, Trang Thi; Hamaï, Ahmed; Hienzsch, Antje; Cañeque, Tatiana; Müller, Sebastian; Wicinski, Julien; Cabaud, Olivier; Leroy, Christine; David, Amandine; Acevedo, Verónica; Ryo, Akihide; Ginestier, Christophe; Birnbaum, Daniel; Charafe-Jauffret, Emmanuelle; Codogno, Patrice; Mehrpour, Maryam; xRodriguez, Raphaël Rodriguez (Oct 2017). "Salinomycin kills cancer stem cells by sequestering iron in lysosomes". Nature Chemistry. 9 (10): 1025–1033. https://doi.org/10.1038/nchem.2778
3. Solier, Stéphanie; Müller, Sebastian; Tatiana, Cañeque; Antoine, Versini; Arnaud, Mansart; Fabien, Sindikubwabo; Leeroy, Baron; Laila, Emam; Pierre, Gestraud; G. Dan, Pantoș; Vincent, Gandon; Christine, Gaillet; Ting-Di, Wu; Florent, Dingli; Damarys, Loew; Sylvain, Baulande; Sylvère, Durand; Valentin, Sencio; Cyril, Robil; François, Trottein; David, Péricat; Emmanuelle, Näser; Céline, Cougoule; Etienne, Meunier; Anne-Laure, Bègue; Hélène, Salmon; Nicolas, Manel; Alain, Puisieux; Sarah, Watson; Mark A., Dawson; Nicolas, Servant; Guido, Kroemer; Djillali, Annane; Raphaël, Rodriguez (2023). "A druggable copper-signalling pathway that drives inflammation". Nature. 617 (7960): 386–394. https://doi.org/10.1038/s41586-023-06017-4
4. Devaraj, Neal K.; Weissleder, Ralph; Hilderbrand, Scott A. (December 2008). "Tetrazine-based cycloadditions: application to pretargeted live cell imaging". Bioconjugate Chem. 19 (12): 2297–2299. https://doi.org/10.1021/bc8004446
5. Ding, H.; Demple, B (2000). "Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator". Proc. Natl. Acad. Sci. U.S.A. 97 (10): 5146–5150. Bibcode:2000PNAS...97.5146D. https://doi.org/10.1073/pnas.97.10.5146
6. Dieterich; et al. (2007). "Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging". Nature Protocols. 2 (3): 532–540. https://doi.org/10.1038/nprot.2007.52
7. Yu; et al. (2012). "Genetically Encoded Cyclopropene Directs Rapid, Photoclick-Chemistry-Mediated Protein Labeling in Mammalian Cells". Angew Chem Int Ed Engl. 51 (42): 10600–10604. https://doi.org/10.1002/anie.201205352
8. (a) Liang, G.; Ren, H.; Rao, J. Nat. Chem. 2010, 2, 54. (b) Ren, H.; Xiao, F.; Zhan, K.; Kim, Y.-P.; Xie, H.; Xia, Z.; Rao, J. Angew.Chem., Int. Ed. 2009, 48, 9658.
9. Ilya A. Osterman; Alexey V. Ustinov; Denis V. Evdokimov; Vladimir A. Korshun; Petr V. Sergiev; Marina V. Serebryakova; Irina A. Demina; Maria A. Galyamina; Vadim M. Govorun; Olga A. Dontsova (January 2013). "A nascent proteome study combining click chemistry with 2DE" (PDF). Proteomics. 13 (1): 17–21. https://doi.org/10.1002/pmic.201200393
10. Michael Floros; Alcides Leão; Suresh Narine (2014). "Vegetable Oil Derived Solvent, and Catalyst Free "Click Chemistry" Thermoplastic Polytriazoles". BioMed Research International. 2014: 1–14. https://doi.org/10.1155/2014/792901
