Procedures and precautions for preparation of mouse thymus single cell suspension
Preparation process of mouse thymus single cell suspension
1. Use the cervical dislocation method to execute the mice, 75% alcohol immersion for 5 min, remove the mice placed on a sterile operating table, abdomen facing up.
2. Cut open the thoracic cavity of the mouse below the sternum, and the white transparent thymus can be seen, which is distributed in two lobes and located in front of the two lungs and directly behind the sternum.
3. Remove the thymus and soak it in clean PBS solution.
4. Place the thymus in a 200 mesh sieve and gently grind with a tissue grinder stick until there are no visible lumps.
5. Rinse the screen with 15 mL of PBS and collect the rinsate in a 15 mL centrifuge tube and centrifuge at 300 g for 5 min, discarding the supernatant.
6. Resuspend thymocytes with cell staining buffer and adjust the cell concentration to 1 × 107/mL.
Mouse thymus FSC/SSC map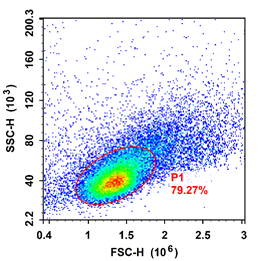
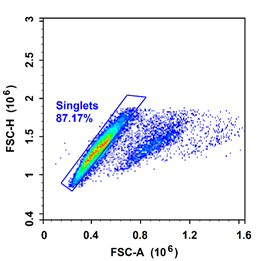
Precautions:
1. Open the thoracic cavity to separate the thymus.
1. Open the thoracic cavity to isolate the thymus, taking care not to cut large blood vessels or break the heart.
2. If necessary, inject 0.1 mL of black ink into the abdominal cavity before executing the mice to darken the lymph nodes in the thoracic cavity for easy identification and removal.
3. 2 × 108 cells are typically obtained from 3- to 6-week-old mice. The thymocytes gradually decreased with the age of the mice.
For more information, visit our website: www.aladdinsci.com.
