Proline-type Organocatalysts
Since the first proline-catalyzed asymmetric synthesis was reported in 1971[1], a number of asymmetric syntheses using proline and derivatives have been developed. Proline-type organocatalysts are predominantly applicable to a wide assortment of asymmetric aldol reactions and asymmetric 1,4-additions, and see further use for the synthesis of natural products. Aladdin offers an assortment of high performing proline-derived organocatalysts that excel furnishing complex intermediates to meet your research needs.
Prolinol Derivatives
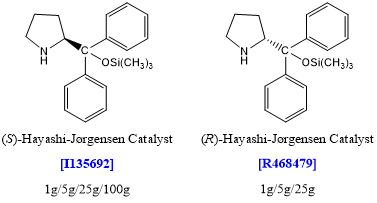

Applications of [I135692][2]
The researchers have developed a new organocatalytic tandem reaction of α,β-unsaturated aldehydes and Nazarov reagent catalyzed by a diarylprolinol ether following a Michael/Morita-Baylis-Hillman mechanism. The reaction proceeds in high enantio- and diastereoselectivity for a wide range of α,β-unsaturated aldehydes and different β-ketoesters. Mechanistic studies indicate that the TMS-protected prolinol also acts as the catalyst in the Morita-Baylis-Hillman reaction, and is the first chiral secondary amine catalyst reported for this reaction. Furthermore, a number of stereoselective transformations have been presented that lead to various types of optically active cyclohexenone and cyclohexanone derivatives with up to four stereocenters.

Applications of [R468479][3]
One-pot operations are effective for carrying out several transformations and forming several bonds in a single pot, while at the same time cutting out several purification steps, minimizing chemical waste generation, and saving time. To simplify the synthesis the researchers investigated the preparation of Tamiflu by a small number of one-pot operations. Their strategy was to construct a key, fully functionalized ethyl cyclohexenecarboxylate intermediate in a single-pot operation as the first step; after this, the remainder of the synthesis consists simply of functional group manipulations, also carried out in one-pot operations. The first key reaction relied on organocatalysis, a relatively new, rapidly developing technology in synthetic organic chemistry.
Proline Derivatives
Applications of [P108709][4]
Studies of carbohydrates have been hampered by the lack of chemical strategies for the expeditious construction and coupling of differentially protected monosaccharides. Here, a synthetic route based on aldol coupling of three aldehydes is presented for the de novo production of polyol differentiated hexoses in only two chemical steps. The dimerization of α-oxyaldehydes, catalyzed by l-proline, is then followed by a tandem Mukaiyama aldol addition-cyclization step catalyzed by a Lewis acid. Differentially protected glucose, allose, and mannose stereoisomers can each be selected, in high yield and stereochemical purity, simply by changing the solvent and Lewis acid used. The reaction sequence also efficiently produces 13C-labeled analogs, as well as structural variants such as 2-amino– and 2-thio–substituted derivatives.
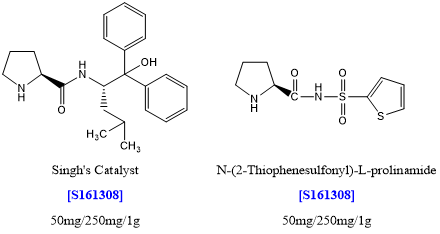
Applications of [S161308][5]
The researchers have successfully reported highly efficient organocatalysts for an asymmetric direct aldol reaction using both cyclic and acyclic ketones and various aldehydes in an aqueous medium. In most of the cases, they have achieved >99% ee by using 0.5 mol % of the catalyst in brine. The added advantage of the catalyst is that it does not require any acid additive to achieve high enantioselectivity.
References
1. U. Eder, G. Sauer, R. Wiechert, Angew. Chem. Int. Ed. 1971, 10, 496. DOI: https://doi.org/10.1002/anie.197104961
2. S. Cabrera, J. Alemán, P. Bolze, S. Bertelsen, K. A. Jørgensen, Angew. Chem. Int. Ed. 2008, 47, 121. DOI: https://doi.org/10.1002/anie.200704076
3. H. Ishikawa, T. Suzuki, Y. Hayashi, Angew. Chem. Int. Ed. 2009, 48, 1304. DOI: https://doi.org/10.1002/anie.200804883
4. A. B. Northrup, D. W. C. MacMillan, Science 2004, 305, 1752. DOI: https://doi.org/10.1126/science.1101710
5. V. Maya, M. Raj, V. K. Singh, Org. Lett. 2007, 9, 2593. DOI: https://doi.org/10.1021/ol071013l