Targeted protein degradation technology: proprietary drugs are not difficult, drug resistance is no longer

Product Manager:Harrison Miller
The anticancer drug Lenalidomide(CAS:191732-72-6)was once one of the world's top-selling small molecule drugs and orphan drugs, with sales of up to 9.978 billion US dollars in 2022, and its development company Celgene made huge profits.Lenalidomide is a derivative of Thalampiperidone(CAS:50-35-1), which was previously banned. Thalampiperidone was stopped from being marketed as an antiemetic drug by the FDA because it can cause limb deformities and other developmental defects in fetuses. Thalampiperidone, lenalidomide, and Pomalidomide(CAS:19171-19-8)were subsequently marketed as anticancer agents with the efforts of Celgene. And how it causes limb deformities and other developmental defects was not revealed until 2010 -- in the form of Molecular glues that induce the degradation of proteins. Thalampiperidone is the earliest Targeted Protein Degredation (TPD) drug.
TPD technology: breakthrough of difficult drugs, no drug resistance
Small molecule drugs have high oral convenience, easy absorption, and high drug utilization. But small molecules have limited scope. Of the nearly 20 000 known genes, only about 3000 are considered therapeuticable and only about 400 are targets of existing drugs, including types such as kinases, receptors, and channel proteins. As the development of conventional targets has been exhausted, the development of drugs targeting difficult targets has attracted much attention. TPD technology uses the natural degradation system in cells to directly degrade proteins, which is expected to fill the gap in solving the problem of patent drugs.
At the same time, almost all targeted anticancer drugs will encounter resistance after a period of clinical use. TPD technology can solve the problem of drug resistance caused by target pocket mutations, bringing hope to patients. Among them, PROTAC and molecular glue are currently the two most mature targeted protein degradation technologies in terms of the extent and heat of research, the number of companies and pipelines, and the progress of clinical trials.
PROTAC: Targeted degradation based on ubiquitination tags
PROTAC degrades the ubiquitination label Protein of interest (POI) by Proteasome. PROTAC consists of three modules: one is a ligand that connects to the pathogenic protein POI, and the other is a ligand that connects to the E3 ligase (labeled ubiquitination). The two are connected by a Linker, and when the E3 ligase and POI are near each other, it is tagged with a string of ubiquitination tags.
PROTAC has a mature design system and a range of proteins that can be targeted. In theory, as long as the target protein is obtained through high-throughput screening, small molecular ligands with high activity can be designed into PROTAC molecules for degradation. PROTAC has been successfully designed for more than 200 targets, and more than 10 PROTAC targets have entered clinical development.
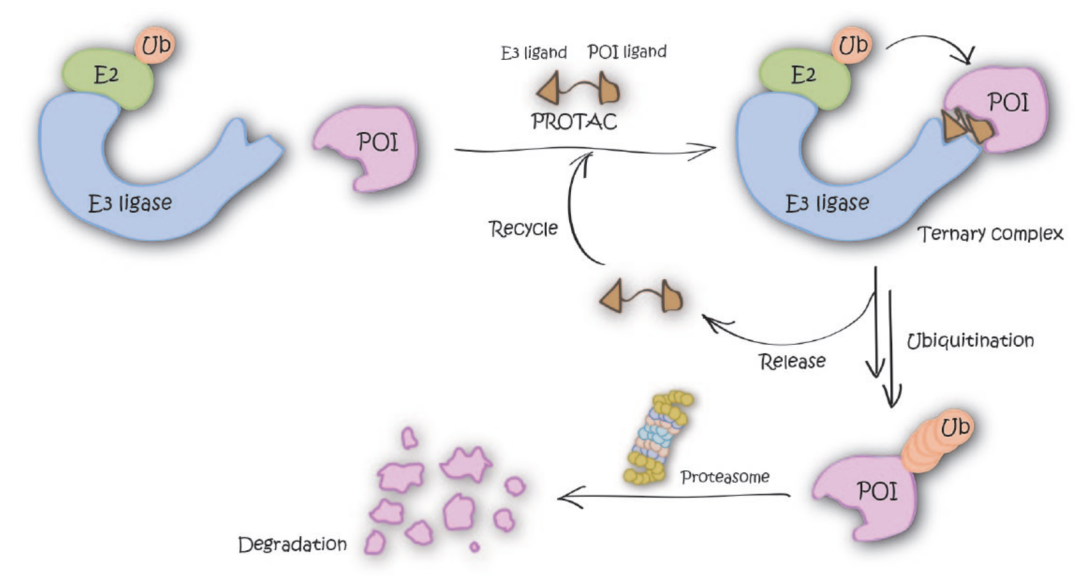
Figure 1: The technical principle of PROTAC1
Well-known refractory targets KRAS and STAT3, low-selective targets such as BET 2/3/4, CDK 2/4/6, KDM4 A/B/C/D protein family, inhibitor resistance targets AR, BTK, EGFR, and IRAK4 targets that have shown insufficient efficacy when used as small molecule inhibitors.These targets, which are difficult to develop in traditional small molecule drugs, have attracted much attention from PROTAC.At present, most of the overseas pharmaceutical enterprises are trying the First-in-Class innovation targets, and the layout is relatively scattered. In order to quickly follow up the development, domestic pharmaceutical enterprises mainly target AR and BTK.
Molecular glue: the "glue" between E3 ligases and substrates
Molecular glue, which acts like glue to E3 ligases and new substrates, can cause protein interactions with proteins that have no natural affinity, hence the name.
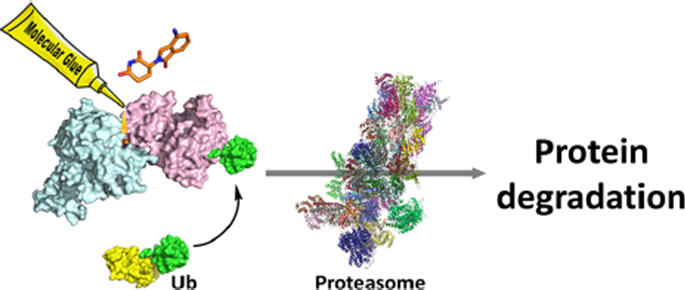
Figure 2: The principle of molecular glue technology
Compared with molecular glue, PROTAC has the advantage of relatively mature modular development methods and a wide range of targets. The disadvantages are poor drug yield and more time required for PK/PD optimization. Although molecular glue has a small molecular weight, it has better drug performance, but at present, it mainly relies on the modification of existing molecules and high-throughput screening to obtain new molecular glue degraders, which cannot be reasonably designed for the target. Therefore, the discovery method of new molecular glue has become the focus of attention in the industry.
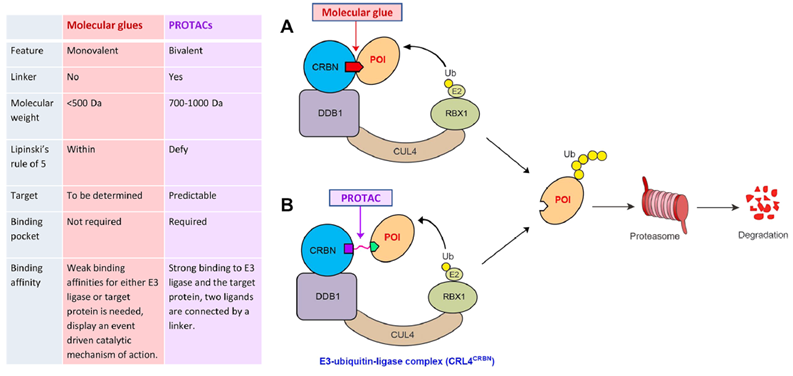
Figure 3: Comparison between PROTAC and molecular glue techniques
For PROTAC, future opportunities lie in finding more E3 ligases and small molecular weight, highly active E3 ligands, broadening the range of targets and indications, incorporating antibody coupling and nanomedicine delivery strategies to improve drug yield and safety, and adopting novel computational tools with predictive capabilities. Compared to PROTAC, once the molecular glue is proof-of-concept, the process from PK/PD optimization, CMC to post-production control will be smoother, and the market potential will likely be greater than that of PROTAC.
Conclusion
Targeted protein degradation drug technology provides a new powerful tool for drug discovery and life science research, and can become an important therapeutic means, providing the potential for innovative treatment options for patients with various diseases. We will continue to develop and provide high-purity, high-efficiency and expanding the scope of drug target ligands and screening reagents, to provide the most reliable help for new drug research and development.
Reference
1. T. Ishida and A. Ciulli, “E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones,” J. SLAS Discovery 26(4), 484-502 (2021). https://doi.org/10.1177/2472555220965528
Aladdin:https://www.aladdinsci.com
