Pyridinium Chlorochromate (PCC) Corey-Suggs Reagent

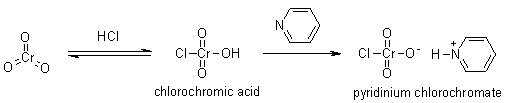
Chlorochromic acid can be prepared by dissolving chromium trioxide in 6 M aqueous hydrochloric acid. When pyridine is added to this solution, pyridinium chlorochromate is formed as orange crystals.
Pyridinium chlorochromate (PCC) exhibits properties that make it a suitable alternative to pyridinium dichromate (PDC). Like PDC, PCC is not highly hygroscopic, is stable, and is readily available commercially for storage. Furthermore, PCC is soluble in a wide range of organic solvents, with dichloromethane being the most commonly used solvent at room temperature. In contrast, the use of dimethylformamide (DMF) as a solvent for PCC has been found to promote over-oxidation of primary alcohols, leading to the formation of carboxylic acids instead of the desired aldehydes or ketones.
While pyridinium chlorochromate (PCC) is more acidic than pyridinium dichromate (PDC), it is still possible to oxidize acid-labile compounds in the presence of buffers such as sodium acetate or carbonates. However, one potential drawback of using PCC is the formation of viscous materials during the oxidation reaction, which can complicate the isolation of the desired product. To simplify the work-up process, additives such as Celite, powdered molecular sieves, or magnesium sulfate can be added to the PCC oxidation reaction mixtures. These additives help to deposit the reduced chromium salts and other reagent-derived byproducts onto their surfaces, allowing them to be easily removed by filtration.
A full review of chromium-based reagents can be found in the book written by Tojo and Fernández (Oxidation of Alcohols to Aldehydes and Ketones, Springer Berlin, 2006, 1-97.).
Attention: Chromium (VI) compounds are toxic and must be handled with care.
Recent Literature

Pyridinium chlorochromate is a readily available, stable reagent, that oxidizes a wide variety of alcohols to carbonyl compounds with high efficiency.
E. J. Corey, J. W. Suggs, Tetrahedron Lett., 1975, 16, 2647-2650.
https://doi.org/10.1016/S0040-4039(00)75204-X

A domino oxidation of primary alcohols gives α,β-unsaturated compounds using the combination of PCC-NaOAc and stabilized Wittig reagents.
J. Shet, V. Desai, S. Tilve, Synthesis, 2004, 1859-1863.
https://doi.org/10.1055/s-2004-829123
A facile and quantitative preparation of carboxylic acids by a pyridinium chlorochromate (PCC) catalyzed (2 mol%) oxidation of primary alcohols and aldehydes using 2.2 equivalents and 1.1 equivalents of H5IO6, respectively, in acetonitrile is described here.
M. Hunsen, Synthesis, 2005, 2487-2490.
https://doi.org/10.1055/s-2005-872085
M. Hunsen, Synthesis, 2005, 2487-2490.
https://doi.org/10.1055/s-2005-872085
A. Barbero, Y. Blanco, C. Garcia, Synthesis, 2000, 1223-1228.
https://doi.org/10.1055/s-2000-6409
Isatins can be synthesized from α-formyl amides in good yields via one-pot intramolecular cyclization-oxidation reaction in the presence of pyridinium chlorochromate (PCC). The reaction proceeded smoothly under air, offers a broad substrate scope, and is operationally simple.
Q. Yue, Y. Wang, L. Hai, L. Guo, H. Yin, Y. Wu, Synlett, 2016, 27, 1292-1296.
https://doi.org/10.1055/s-0035-1561372
A multifunctional modular organocatalysis enables a simple and efficient approach to enantioenriched α,β-disubstituted γ-butyrolactones via a one-pot sequential Michael-hemiacetalization-oxidation reaction. The catalytic process offers good substrate compatibility, and the products can be transformed to synthetically useful molecules.
P. Mahto, N. K. Rana, K. Shukla, B. G. Das, H. Joshi, V. K. Singh, Org. Lett., 2019, 21, 5962-5966
https://doi.org/10.1021/acs.orglett.9b02094
Quoted from:https://www.organic-chemistry.org/chemicals/oxidations/pyridinium-chlorochromate-pcc.shtm
Aladdin:https://www.aladdinsci.com



