RAFT Polymerization
Sandra Forbes
Product Manager
Introduction
RAFT (Reversible Addition-Fragmentation Chain Transfer) polymerization is a type of reversible deactivation radical polymerization (RDRP) known for imparting living characteristics to radical polymerization. Its historical development was pioneered at CSIRO. The key advantages of RAFT polymerization include:
· The ability to control the polymerization of most monomers typically polymerized via radical methods, such as (meth)acrylates, (meth)acrylamides, acrylonitrile, styrenes, dienes, and vinyl monomers.
· Tolerance of functional groups in monomers and solvents without the need for protection (e.g., OH, NR2, COOH, CONR2, SO3H), allowing polymerization in aqueous or protic media.
· Compatibility with a range of reaction conditions, including bulk, organic or aqueous solutions, emulsions, mini-emulsions, and suspensions.
· Ease of implementation and cost-effectiveness compared to other technologies.
In an ideal living polymerization, all polymer chains are initiated at the start, grow uniformly, and remain active throughout the process, without irreversible chain transfer or termination. When initiation is fast relative to propagation, the molecular weight distribution remains narrow, and chains can be extended by adding more monomers. In traditional radical polymerization, not all chains are active simultaneously. However, in RAFT polymerization, reversible deactivation of propagating radicals occurs through specific reagents, ensuring that most chains remain dormant. A rapid equilibrium is maintained between active and dormant chains, enabling precise control over polymerization (Figure 1).
![]()

Figure 1. RAFT Polymerization Schematic. The proportions of chain types illustrated here do not reflect those expected in a well-designed experiment. In an ideal scenario, all living chains grow at the same rate and maintain equal chain lengths, as the equilibrium between dormant and active chain ends occurs rapidly compared to propagation. The RAFT agent is symbolized as ′ZC(=S)S′.
Under these conditions, molecular weights can increase linearly with monomer conversion, and the molecular weight distributions can remain very narrow (Figure 2). Most of the polymerization product will consist of dormant chains.

Figure 2. Typical molecular weight distributions for both conventional and RAFT polymerizations of styrene under comparable experimental conditions.
The mechanism of chain activation and deactivation in RAFT polymerization is illustrated in Figure 3. These RAFT equilibria reactions occur alongside the standard processes of initiation, propagation, and termination seen in conventional radical polymerization. Ideally, the RAFT agent acts as a transfer agent, but termination is not prevented in the RAFT process. The retention of thiocarbonylthio groups in the polymer product imparts the living character to RAFT polymerization, making it suitable for synthesizing block copolymers and end-functional polymers. For certain applications, removal or modification of the thiocarbonylthio group may be necessary, and several methods for end-group removal have been developed that can be easily integrated into polymer synthesis.

Figure 3. Mechanism for reversible addition-fragmentation chain transfer (RAFT)
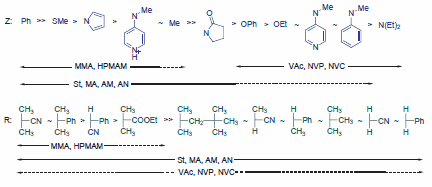
Selecting the appropriate RAFT agent (ZC(=S)SR) for the monomers and reaction conditions is critical to the success of a RAFT polymerization experiment, though the process is manageable. The efficiency of RAFT agents is determined by the R and Z substituents, and selection guidelines have been established (Figure 3). Using one of two RAFT agent classes, most monomers can be well-controlled, minimizing retardation and ensuring a high fraction of living chains. The first class is suitable for more activated monomers (MAMs) like methacrylics (e.g., methyl methacrylate, methacrylic acid, hydroxypropyl methacrylamide) and acrylics (e.g., methyl acrylate, acrylic acid, acrylamide, acrylonitrile, styrene). The second class is designed for less activated monomers (LAMs) such as vinyl acetate, N-vinylpyrrolidone (NVP), or N-vinylcarbazole (NVC). Recently, a switchable RAFT agent has been introduced that can control the polymerization of both MAMs and LAMs. Specific end-functionalities or polymer architectures may require alternative RAFT agents.
Figure 4. Guidelines for selecting RAFT agents (Z-C(=S)S-R) for different polymerizations: For the ‘Z’ group, the rates of addition and transfer constants decrease, while fragmentation rates increase from left to right. For the ‘R’ group, fragmentation rates decrease from left to right. A dashed line indicates limited control, such as potential retardation or high dispersity.
With the right selection of reagents and polymerization conditions, RAFT polymerization can be employed to synthesize well-defined homo, gradient, diblock, triblock, and star polymers, as well as more complex structures like microgels and polymer brushes. Current applications span a wide range, including novel surfactants, dispersants, coatings, adhesives, biomaterials, membranes, drug delivery systems, electroactive materials, and other areas within nanotechnology.
RAFT Polymerization of 'More-Activated Monomers' (MAMs)
Trithiocarbonates (Z=S-alkyl) provide good control over the polymerization of MAMs, with Z preferably derived from a low-volatility thiol. Aromatic dithioesters (Z=aryl) are among the most active RAFT agents and generally perform well in MAM polymerizations. However, aromatic-substituted RAFT agents can cause retardation at high concentrations and are more prone to hydrolysis and decomposition in the presence of Lewis acids. If hydrolysis is a concern, alkyl-substituted RAFT agents may be a better choice. Bis(thiocarbonyl) disulfides are useful precursors for tertiary RAFT agents and can form RAFT agents in situ during polymerization.
The R group must efficiently reinitiate polymerization and act as a good homolytic leaving group relative to the propagating radical. R⋅ must also reinitiate polymerization quickly, adding to the monomer at a rate faster than propagation. For acrylates and acrylamides, cyanomethyl is a good choice for the R group. In the case of methacrylates, the selection of R is critical, with some of the most effective RAFT agents using tertiary cyanoalkyl groups.
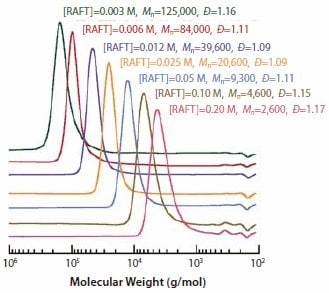
An example demonstrating the utility of the RAFT process involves the polymerization of methyl methacrylate (MMA). A series of MMA polymerizations with high conversions (80-100%) were conducted at 90 oC using 1,1'-azobis(1-cyclohexanecarbonitrile) as the initiator and a ~60-fold range of S-dodecyl S-(2-cyano-4-carboxy)but-2-yl trithiocarbonate concentrations. After six hours, the molecular weight distributions ranged from 2,600 to 125,000, consistent with the concentrations of RAFT agent and initiator. All samples showed narrow molecular weight distributions (PDI <1.2), as illustrated in Figure 5.
Figure 5. Molecular weight distributions for PMMA obtained from high-conversion RAFT polymerization of MMA (6.55 M in benzene) using 1,1’-azobis(1-cyclohexanecarbonitrile) (0.0018 M) as the initiator and varying concentrations of RAFT agent 5 over 6 hours at 90 °C.
RAFT Polymerization of 'Less-Activated Monomers' (LAMs)
Less active RAFT agents, such as those with Z=NR’2 (dithiocarbamates) or Z=OR’ (xanthates) where R’ is alkyl or aryl, provide good control over polymerization. More active RAFT agents, including those with Z=R (dithioesters) or SR (trithiocarbonates), can inhibit the polymerization of less activated monomers (LAMs). The choice of the R group is also crucial, as most monomers in this category have high propagation rate constants, leading to potential inhibition periods due to slow reinitiation. Examples of vinyl acetate (VAc) polymerization are provided in Table 1.
Table 1. RAFT Polymerization of Vinyl Acetate
Switchable RAFT Agents
We recently introduced a new class of stimuli-responsive RAFT agents that can be “switched” to control the polymerization of both MAMs and LAMs, offering a more convenient method for producing polyMAM-block-polyLAM polymers with narrowed molecular weight distributions. This approach was demonstrated using 4-pyridinyl-N-methyldithiocarbamate derivatives to synthesize PMMA-block-PVAc and PMA-block-PNVC. The N-4-pyridinyl-N-methyldithiocarbamates effectively control the polymerization of LAMs (Figure 6) and, when protonated, also provide excellent control over MAM polymerization.
Figure 6. RAFT Agent capable of polymerization of both LAMs and MAMs controlled by pH.
Conclusions
Reversible Addition Fragmentation chain Transfer (RAFT) has become one of the leading techniques for controlling radical polymerization. It is known for its robustness and versatility, making it applicable to most monomers involved in radical polymerization. However, achieving successful polymerization requires careful selection of the appropriate RAFT agent for the specific monomers, as well as optimizing the reaction conditions.
References
1. Moad G, Rizzardo E, Thang SH. 2005. Living Radical Polymerization by the RAFT Process. Aust. J. Chem.. 58(6):379. https://doi.org/10.1071/ch05072
2. Moad G, Rizzardo E, Thang SH. 2006. Living Radical Polymerization by the RAFT Process-A First Update. Aust. J. Chem.. 59(10):669. https://doi.org/10.1071/ch06250
3. Moad G, Rizzardo E, Thang SH. 2008. Radical addition-fragmentation chemistry in polymer synthesis. Polymer. 49(5):1079-1131. https://doi.org/10.1016/j.polymer.2007.11.020
4. Moad G, Rizzardo E, Thang SH. 2008. Toward Living Radical Polymerization. Acc. Chem. Res.. 41(9):1133-1142. https://doi.org/10.1021/ar800075n
5. Moad G, Rizzardo E, Thang SH. 2009. Living Radical Polymerization by the RAFT Process - A Second Update. Aust. J. Chem.. 62(11):1402. https://doi.org/10.1071/ch09311
6. Moad G, Chiefari J, Krstina J, Postma A, Mayadunne RTA, Rizzardo E, Thang S. 2000. H. Polym. Int.. 49993-1001.
7. Rizzardo E, Chiefari J, Mayadunne RTA, Moad G, Thang SH. 2000. Synthesis of Defined Polymers by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process.278-296. https://doi.org/10.1021/bk-2000-0768.ch020
8. Benaglia M, Chen M, Chong YK, Moad G, Rizzardo E, Thang SH. 2009. Polystyrene-block-poly(vinyl acetate) through the Use of a Switchable RAFT Agent. Macromolecules. 42(24):9384-9386. https://doi.org/10.1021/ma9021086
9. Benaglia M, Chiefari J, Chong YK, Moad G, Rizzardo E, Thang SH. 2009. Universal (Switchable) RAFT Agents. J. Am. Chem. Soc.. 131(20):6914-6915. https://doi.org/10.1021/ja901955n
10. Moad G, Chong Y, Postma A, Rizzardo E, Thang SH. 2005. Advances in RAFT polymerization: the synthesis of polymers with defined end-groups. Polymer. 46(19):8458-8468. https://doi.org/10.1016/j.polymer.2004.12.061
11. Moad G, Mayadunne RT, Rizzardo E, Skidmore M, Thang SH. 2003. Synthesis of novel architectures by radical polymerization with reversible addition fragmentation chain transfer (RAFT polymerization). Macromol. Symp.. 192(1):1-12. https://doi.org/10.1002/masy.200390029
12. Chong YK, Moad G, Rizzardo E, Thang SH. 2007. Thiocarbonylthio End Group Removal from RAFT-Synthesized Polymers by Radical-Induced Reduction. Macromolecules. 40(13):4446-4455. https://doi.org/10.1021/ma062919u
13. Postma A, Davis TP, Evans RA, Li G, Moad G, O'Shea MS. 2006. Synthesis of Well-Defined Polystyrene with Primary Amine End Groups through the Use of Phthalimido-Functional RAFT Agents. Macromolecules. 39(16):5293-5306. https://doi.org/10.1021/ma060245h
14. Postma A, Davis TP, Li G, Moad G, O'Shea MS. 2006. RAFT Polymerization with Phthalimidomethyl Trithiocarbonates or Xanthates. On the Origin of Bimodal Molecular Weight Distributions in Living Radical Polymerization. Macromolecules. 39(16):5307-5318. https://doi.org/10.1021/ma0604338
15. Postma A, Davis TP, Moad G, O'Shea MS. 2005. Thermolysis of RAFT-Synthesized Polymers. A Convenient Method for Trithiocarbonate Group Elimination. Macromolecules. 38(13):5371-5374. https://doi.org/10.1021/ma050402x
16. Chong B, Moad G, Rizzardo E, Skidmore M, Thang SH. 2006. Thermolysis of RAFT-Synthesized Poly(Methyl Methacrylate). Aust. J. Chem.. 59(10):755. https://doi.org/10.1071/ch06229
17. Rizzardo E, Chen M, Chong B, Moad G, Skidmore M, Thang SH. 2007. RAFT Polymerization: Adding to the Picture. Macromol. Symp.. 248(1):104-116. https://doi.org/10.1002/masy.200750211
18. Chong YK, Moad G, Rizzardo E, Skidmore MA, Thang SH. 2007. Reversible Addition Fragmentation Chain Transfer Polymerization of Methyl Methacrylate in the Presence of Lewis Acids: An Approach to Stereocontrolled Living Radical Polymerization. Macromolecules. 40(26):9262-9271. https://doi.org/10.1021/ma071100t
19. Thang SH, Chong, Mayadunne RT, Moad G, Rizzardo E. 1999. A novel synthesis of functional dithioesters, dithiocarbamates, xanthates and trithiocarbonates. Tetrahedron Letters. 40(12):2435-2438. https://doi.org/10.1016/s0040-4039(99)00177-x
20. Chong YK, Krstina J, Le TPT, Moad G, Postma A, Rizzardo E, Thang SH. 2003. Thiocarbonyl Compounds [SC(Ph)S-R] in Free Radical Polymerization with Reversible Addition-Fragmentation Chain Transfer (RAFT Polymerization). Role of the Free-Radical Leaving Group (R). Macromolecules. 36(7):2256-2272. https://doi.org/10.1021/ma020882h
Aladdinsci: https://www.aladdinsci.com/