Realizing High Efficiency in Organic Light Emitting Devices

INTRODUCTION
The electroluminescence effect of organic molecules has been a well-known phenomenon for more than 50 years.1,2 However, it was not until the late 1980s that it showed any promise of practical application. Successful application of organic luminescence in light-emitting devices requires device structures that overcome the problems associated with the high resistivity of organic materials, and it also requires the realization of well-balanced charge injection from the electrodes to the organics.Tang and van Slyke3 addressed both of these problems with the concept of thin-film heterostructures for organic LEDs (OLEDs). Figure 1 shows a schematic of a dual heterostructure OLED consisting of three organic layers sandwiched between electrodes. The organic layers adjacent to the cathode and anode are the electron transport layer (ETL) and hole transport layer (HTL), respectively.
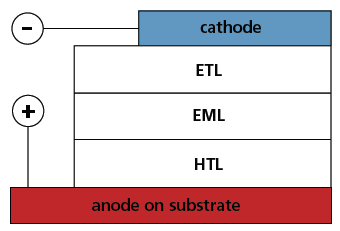
Figure 1.Schematic of a double heterostructure OLED consisting of a hole transport layer (HTL), electron transport layer (ETL), emissive layer (EML), and the electrodes.
During OLED operation, holes and electrons injected through the opposing electrodes are transported to the emissive layer (EML) where they complex to form excitons. Film thicknesses of 500 Å or less can reduce the drive voltage to levels as low as 5-10 V, and the separated hole and electron conducting layers provide efficient charge injection and carrier complexation. This leads to exciton formation and eventual emission as the exciton decays to the ground state. Shortly after the introduction of OLEDs based on thin-film heterostructures, it was shown that a two-component emissive layer doped with an emitter molecule in an appropriate substrate increased the level of charge complexation and exciton confinement in the emissive layer, resulting in improved device efficiency. This also eliminated the self-detonation of the emitting dopant.4
INCREASING EFFICIENCY OF OLEDs USING PHOSPHORESCENCE
The holes and electrons in OLEDs are odd electron species with a uniform distribution of ms=±½. When holes and electrons recombine to form excitons, a statistical mixture of singlet and triplet excitons is formed.5,6 This results in a population of 25% singlet excitons and 75% triplet excitons and has significant implications for OLED efficiency.7 Most of the emitting dopants developed for OLEDs prior to the late 1990s emitted from fluorescent states, which only affected the formed This limited the internal quantum efficiency of fluorescence-based devices to 25%, corresponding to an external efficiency of only about 5% (positive). In the late 1990s, a new family of emitting dopants was introduced that significantly improved the efficiency of OLEDs. Key to this efficiency improvement was the realization that the triplet exciton fraction is more important than the single-line state. Efficient trapping of triplet excitons requires phosphorescent dopants that trap both singlet and triplet excitons. Another requirement for phosphorescent dopants is that they have a radiation lifetime comparable to the RC time constant of the OLED, which is typically in the microsecond range. The best way to achieve high phosphorescence efficiencies and microsecond radiative lifetimes is to incorporate heavy metal atoms into the dopant, whose spin-orbit coupling will effectively facilitate intersystem crossing between single and triple-linear states. The most commonly used metal for this purpose is Ir, however, effective phosphorescent dopants have also begun to be prepared using other heavy metals, including Pt, Ru, Re, Au, and Os.
Figure 2 shows the structures and CIE chromaticity coordinates of some Ir-based organometallic dopants. Among them, OLEDs have been prepared with four circled dopants; their CIE coordinates have been marked with colored arrows, respectively. Since the introduction of Ir-based phosphors in OLEDs in 1999,8 nearly 200 different Ir complexes have been doped into OLEDs, most of them with external efficiencies of 8% or higher.9 Several groups have reported that external efficiencies of >20% can be obtained using Ir-based materials in optimized devices, corresponding to internal efficiencies close to 100%.10-12

Figure. 2. Chemical structure of organic light-emitting diodes, CIE chromaticity coordinates and phosphorescence spectra of iridium ring metallization complexes.
As the emission energy of organometallic phosphors depends on the structure of organic ligands, it is possible to design phosphorescent emitters that cover most of the visible spectrum.13,14 Transition metal complexes emit from their lowest energy triplet excited state, which can also be fine-tuned by the metal center.This state is primarily observed on the cyclometalating ligands, mixed with singlet metal-to-ligand charge transfer (1MLCT). Modification of ancillary (“non-emissive”) ligands affects the energy of the metal orbitals and thus the amount of 1MLCT character in the excited state. Varying the ratio of ligand centered to 1MLCT character directly affects the energy of the mixed excited state.13 By altering the ancillary ligand in (F2ppy)2Ir(L^X) complexes (where L^X represents the ancillary ligand), it becomes feasible to change the emission energy of the complex from 458 to 512 nm.[(F2ppy)2Ir(pz2Bpz2)], one of these deep blue complexes, has been used to fabricate OLEDs with external efficiencies above 11%.15
APPLICATION OF OLEDS IN ILLUMINATION
Illumination is one of the most important potential applications for LEDs . In contrast to the monochromatic OLEDs described above, devices that serve as illumination sources have slightly different requirements. To be suitable for RGB displays, OLEDs must have a relatively narrow lineshape centered on the peak wavelength.A source of illumination, however, needs to have a broad lineshape and roughly equal intensity across every visible wavelength in order to approximate the blackbody solar spectrum. To achieve complete coverage across the visible spectrum, an OLED used for illumination purposes typically employs multiple emitters that are either co-deposited into a single emissive layer or distributed into different layers or regions of the device.Various device architectures have been reported to achieve efficient white electroluminescence.
It is common for white organic LEDs (WOLEDs) to use several different colored emitters so that the combined output is uniform throughout the visible spectrum .It has been reported that WOLEDs can have fewer than three distinct emitters, but the most common approach is to utilize three, i.e. blue, green, and red.So one of the simplest device architectures involves mixing blue, green and red dopants into a single emissive layer, that the sum of the three emission spectra covers the visible spectrum.16-18 Using phosphorescent emitters in a triple-doped emissive layer can result in highly efficient devices.When using three dopants in a single layer, energy readily transfers from the blue dopant to the green dopant and from the green dopant to the red dopant.To achieve a well-balanced emission color, doping levels in the ratio blue > green >> red must be adjusted carefully .The doping level of the red dopant needs to be lower than 1% to get well-balanced white emission.
To address the issue of inter-dopant energy transfer, a possible approach is to separate the dyes into distinct layers.This stacked concept has been used to prepare efficient WOLEDs with fluorescent or phosphorescent emitters. 18-21Simplified configurations have also been proposed, employing dual-component fluorescent emitters in blue and orange, incorporated into distinct layers.19,22 Though segregating the emitters into separate layers addresses energy transfer issues, it can complicate the device architecture considerably by introducing challenges in achieving balanced carrier recombination and exciton confinement within each of the emitting layers.
The utilization of planar platinum-based dopants enables the creation of a broad-spectrum emitting (white) OLED using a single dopant, in contrast to the previously mentioned methods that relied on two or three distinct emitters. Figure 3 illustrates how this white emission is attained by combining the output from the monomeric (blue) and aggregated (yellow-to-red) states of the same organometallic platinum complex, resulting in an emission spectrum that encompasses the entire visible light range. The balance between monomeric and aggregated emissions can be regulated through both the doping concentration and the steric size of the dopant.23 Increasing the steric size of the dopant hinders aggregate formation, while higher dopant concentrations promote it. Reducing the number of dopants significantly simplifies the device design. Recent research has demonstrated that devices employing the monomer-aggregate approach for broad-spectrum emission can achieve external efficiencies in the range of 15–20%.24,25
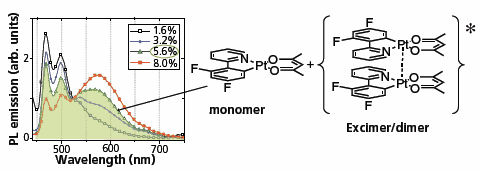
Figure 3. Depicts the photoluminescence spectra of doped films containing F2-ppyPt(acac), showcasing how the spectral profile varies with changes in doping levels. These spectra comprise emission components from both aggregates and monomers. When the doping concentration reaches 5.6%, a balance is achieved in the film between the monomeric and aggregated forms of F2-ppyPt(acac), resulting in the generation of white light. The chemical structures of F2-ppyPt(acac) and its dimer are presented on the right for reference.
White light predominantly consists of around 25% blue, with the remainder encompassing the spectrum between green and red. Interestingly, the excitons, formed when holes and electrons recombine within the OLED, occur in a ratio of 25% singlets to 75% triplets. This resemblance between the blue component of white light and the singlet fraction suggests an alternative strategy for achieving highly efficient white emission. It involves pairing singlet excitons with a blue fluorescent dopant and triplets with phosphors that cover the green and red portions of the spectrum. This approach, combining both fluorescent and phosphorescent emissions, offers several advantages.The introduction of a stable fluorescent blue component is anticipated to address a well-known issue related to the limited operational lifespan of the blue components in WOLEDs. In the quantum efficiency-current density plots of typical three-component phosphorescent WOLEDs, there is often a rapid decline in efficiency at higher current densities soon after reaching its peak.26 This drop in efficiency is attributed to triplet-triplet annihilation, which occurs at elevated currents. In the combined fluorescent and phosphorescent device, this undesired efficiency decrease is mitigated since the triplets are less concentrated in the middle of the emissive layer than near the ETL or HTL interface, where they are initially formed.
A significant breakthrough has been recently reported for phosphorescent-based WOLEDs. Nakayama and colleagues have developed a WOLED that utilizes blue, green, and red phosphors to create a comprehensive white OLED spectrum.27 Their device achieved an impressive efficiency of 64 lm/W at a brightness level of 1000 cd/m². Notably, this efficiency surpasses that of compact fluorescent sources and approaches the efficiency of fluorescent tube sources, which typically range from 75 to 90 lm/W. Furthermore, this device demonstrated an extended operational lifetime exceeding 10,000 hours at this level of brightness. These values represent more than twice the efficiency of previous OLED records, providing clear evidence that OLEDs have a promising future in lighting applications.
CONCLUSION
OLEDs hold great potential for making a significant impact in full-color displays and lighting applications. These applications demand high efficiency, long lifetimes, cost-effective manufacturing, a broad color spectrum for various device sets, and vibrant color saturation. OLEDs have proven their capability in delivering these essential characteristics; nevertheless, large-scale production remains a formidable challenge, resulting in relatively high manufacturing costs. Additionally, one of the technological hurdles lies in achieving extended lifetimes for deep blue OLED devices. Numerous stable red and green phosphorescent emitters have been developed, with device lifetimes nearing 106 hours. In contrast, the operational stability of blue phosphor-based OLEDs is notably shorter, with the best values ranging between 15,000 and 20,000 hours. The exact reasons behind the increased instability of these blue devices remain an open question. While many fluorescent and phosphorescent OLEDs have already found commercial success in smaller mobile displays, there remains ample opportunity for scientific exploration to gain a deeper understanding of the parameters that govern and limit organic electroluminescence.
REFERENCE
1. Pope M, Kallmann HP, Magnante P. 1963. Electroluminescence in Organic Crystals. The Journal of Chemical Physics. 38(8):2042-2043. https://doi.org/10.1063/1.1733929
2. Bernanose A, Comte M, Vouaux P. 1953. Sur un nouveau mode d'émission lumineuse chez certains composés organiques. J. Chim. Phys.. 5064-68. https://doi.org/10.1051/jcp/1953500064
3. Tang CW, VanSlyke SA. 1987. Organic electroluminescent diodes. Appl. Phys. Lett.. 51(12):913-915. https://doi.org/10.1063/1.98799
4. Tang CW, VanSlyke SA, Chen CH. 1989. Electroluminescence of doped organic thin films. Journal of Applied Physics. 65(9):3610-3616. https://doi.org/10.1063/1.343409
5. Segal M, Baldo MA, Holmes RJ, Forrest SR, Soos ZG. Excitonic singlet-triplet ratios in molecular and polymeric organic materials. Phys. Rev. B. 68(7): https://doi.org/10.1103/physrevb.68.075211
6. Baldo MA, O?Brien DF, Thompson ME, Forrest SR. Excitonic singlet-triplet ratio in a semiconducting organic thin film. Phys. Rev. B. 60(20):14422-14428. https://doi.org/10.1103/physrevb.60.14422
7. Shoustikov A, Yujian You, Thompson M. 1998. Electroluminescence color tuning by dye doping in organic light-emitting diodes. IEEE J. Select. Topics Quantum Electron.. 4(1):3-13. https://doi.org/10.1109/2944.669454
8. Baldo MA, Lamansky S, Burrows PE, Thompson ME, Forrest SR. 1999. Very high-efficiency green organic light-emitting devices based on electrophosphorescence. Appl. Phys. Lett.. 75(1):4-6. https://doi.org/10.1063/1.124258
9. Crabtree R, Mingos D. 2007. Comprehensive Organometallic Chemistry III. 12. Oxford, UK: Elsevier.
10. Tsuboyama A, Iwawaki H, Furugori M, Mukaide T, Kamatani J, Igawa S, Moriyama T, Miura S, Takiguchi T, Okada S, et al. 2003. Homoleptic Cyclometalated Iridium Complexes with Highly Efficient Red Phosphorescence and Application to Organic Light-Emitting Diode. J. Am. Chem. Soc.. 125(42):12971-12979. https://doi.org/10.1021/ja034732d
11. Soichi W, Yuya A, Daisaku T, Junji K. 2005. High-Efficiency Phosphorescent OLEDs using Chemically Doped Layers. J. Photopol. Sci. Technol.. 18(1):83-86. https://doi.org/10.2494/photopolymer.18.83
12. Meerheim R, Walzer K, Pfeiffer M, Leo K. 2006. Ultrastable and efficient red organic light emitting diodes with doped transport layers. Appl. Phys. Lett.. 89(6):061111. https://doi.org/10.1063/1.2268354
13. Li J, Djurovich PI, Alleyne BD, Yousufuddin M, Ho NN, Thomas JC, Peters JC, Bau R, Thompson ME. 2005. Synthetic Control of Excited-State Properties in Cyclometalated Ir(III) Complexes Using Ancillary Ligands. Inorg. Chem.. 44(6):1713-1727. https://doi.org/10.1021/ic048599h
14. Brooks J, Babayan Y, Lamansky S, Djurovich PI, Tsyba I, Bau R, Thompson ME. 2002. Synthesis and Characterization of Phosphorescent Cyclometalated Platinum Complexes. Inorg. Chem.. 41(12):3055-3066. https://doi.org/10.1021/ic0255508
15. Holmes RJ, D?Andrade BW, Forrest SR, Ren X, Li J, Thompson ME. 2003. Efficient, deep-blue organic electrophosphorescence by guest charge trapping. Appl. Phys. Lett.. 83(18):3818-3820. https://doi.org/10.1063/1.1624639
16. Kawamura Y, Yanagida S, Forrest SR. 2002. Energy transfer in polymer electrophosphorescent light emitting devices with single and multiple doped luminescent layers. Journal of Applied Physics. 92(1):87-93. https://doi.org/10.1063/1.1479751
17. Tasch S, List EJW, Ekström O, Graupner W, Leising G, Schlichting P, Rohr U, Geerts Y, Scherf U, Müllen K. 1997. Efficient white light-emitting diodes realized with new processable blends of conjugated polymers. Appl. Phys. Lett.. 71(20):2883-2885. https://doi.org/10.1063/1.120205
18. Kido J, Shionoya H, Nagai K. 1995. Single?layer white light?emitting organic electroluminescent devices based on dye?dispersed poly(N?vinylcarbazole). Appl. Phys. Lett.. 67(16):2281-2283. https://doi.org/10.1063/1.115126
19. Jiang X, Zhang Z, Zhao W, Zhu W, Zhang B, Xu S. 2000. White-emitting organic diode with a doped blocking layer between hole- and electron-transporting layers. J. Phys. D: Appl. Phys.. 33(5):473-476. https://doi.org/10.1088/0022-3727/33/5/301
20. Ko CW, Tao YT. 2001. Bright white organic light-emitting diode. Appl. Phys. Lett.. 79(25):4234-4236. https://doi.org/10.1063/1.1425454
21. D’Andrade B, Thompson ME, Forrest S. 2002. Controlling Exciton Diffusion in Multilayer White Phosphorescent Organic Light Emitting Devices. Advanced Materials. 14147.
22. Yang J, Jin Y, Heremans P, Hoefnagels R, Dieltiens P, Blockhuys F, Geise H, Van der Auweraer M, Borghs G. 2000. White light emission from a single layer organic light emitting diode fabricated by spincoating. Chemical Physics Letters. 325(1-3):251-256. https://doi.org/10.1016/s0009-2614(00)00619-9
23. Adamovich V, Brooks J, Tamayo A, Alexander AM, Djurovich PI, D'Andrade BW, Adachi C, Forrest SR, Thompson ME. 2002. High efficiency single dopant white electrophosphorescent light emitting diodesElectronic supplementary information (ESI) available: emission spectra as a function of doping concentration for 3 in CBP, as well as the absorption and emission spectra of Irppz, CBP and mCP. See http://www.rsc.org/suppdata/nj/b2/b204301g/. New J. Chem.. 26(9):1171-1178. https://doi.org/10.1039/b204301g
24. Cocchi M, Kalinowski J, Virgili D, Fattori V, Develay S, Williams JAG. 2007. Single-dopant organic white electrophosphorescent diodes with very high efficiency and its reduced current density roll-off. Appl. Phys. Lett.. 90(16):163508. https://doi.org/10.1063/1.2722675
25. Williams E, Haavisto K, Li J, Jabbour G. 2007. Excimer-Based White Phosphorescent Organic Light-Emitting Diodes with Nearly 100?% Internal Quantum Efficiency. Adv. Mater.. 19(2):197-202. https://doi.org/10.1002/adma.200602174
26. Sun Y, Giebink NC, Kanno H, Ma B, Thompson ME, Forrest SR. 2006. Management of singlet and triplet excitons for efficient white organic light-emitting devices. Nature. 440(7086):908-912. https://doi.org/10.1038/nature04645
27. Nakayama T, Hiyama K, Furukawa K, Ohtani H. 2007. 19.1:Invited Paper: Development of Phosphorescent White OLED with Extremely High Power Efficiency and Long Lifetime. 38(1):1018-1021. https://doi.org/10.1889/1.2785478
