Stimulus-responsive materials for intelligent drug delivery systems

Sandra Forbes
Product Manager
The rapid development of materials science has profoundly reshaped the core paradigm of drug delivery, particularly exerting unprecedented transformative power in the field of cancer therapeutics. Currently, scientists have successfully developed intelligent systems capable of delivering therapeutic molecules precisely on demand by delicately modulating the physicochemical properties of delivery systems. This innovation significantly enhances the precision and efficacy of treatments. These design breakthroughs are deeply rooted in an increasingly profound understanding of the complexity of the tumor microenvironment, inspired by studies within tumor biology that reveal subtle differences within this microenvironment.
Cancer, as a highly complex disease state, involves intricate interactions among multiple cell types, extracellular matrix components, immune regulatory factors, signaling pathways, and physiological and pathological processes. The extreme diversity of cell types poses significant barriers to targeted drug delivery for cancer treatment. Additionally, the frequent occurrence of multidrug resistance (MDR) in traditional chemotherapy further exacerbates clinical challenges, leading to suboptimal therapeutic outcomes.
The tumor microenvironment, a unique ecosystem characterized by abnormal vascular structures, lymphatic circulation barriers, hypoxic conditions, low pH gradients, distinctive redox environments, high interstitial pressure, and elevated protease activity, further complicates treatment. At the microscopic level, tumors consist not only of cancer cells but also a complex network of stromal cells, endothelial cells, various immune cells, and cancer stem cells (CSCs), creating an internal heterogeneity that provides survival advantages to tumors and promotes their growth, dissemination, and distant metastasis.
However, these seemingly adverse heterogeneous features present opportunities for the design of intelligent polymer systems. By precisely recognizing and responding to subtle differences within the tumor microenvironment, these intelligent systems can devise highly tumor-specific therapeutic strategies, effectively overcoming the limitations of traditional therapies and opening new avenues for cancer treatment. The advancement in this field not only showcases the deep integration of materials science and biomedical engineering but also provides robust technical support for the future innovation of cancer treatment modalities.
Internal regulatory system
Internal regulatory carriers, as a self-adaptive and closed-loop regulation mechanism, can acutely respond to changes in the internal environment, such as fluctuations in pH values, shifts in redox states, enhanced protease activity, or the presence of other biological factors, thereby intelligently regulating the drug release process. When specific alterations occur in the biological environment of the diseased site, these changes trigger corresponding chemical or physical transformations within the delivery system, ensuring that the therapeutic payload is precisely released at the appropriate time and location.
The core of this release mechanism lies in its high degree of autonomy and direct dependence on the physiological state of the disease site, meaning that the drug release profile is entirely shaped by the intrinsic conditions of the lesion area rather than directly controlled by external interventions. Consequently, internal regulatory carriers offer a more precise, flexible, and adaptable solution for drug delivery, contributing to enhanced therapeutic efficacy and reduced side effects.
pH-Responsive System
The pH-responsive system ingeniously harnesses the significant pH differences among various regions within the body, such as the gastrointestinal tract, tumor microenvironment, and intracellular endosomal/lysosomal compartments (as depicted in Figure 1), to achieve localized and precise drug delivery. Cancer cells tend to adopt aerobic glycolysis as their primary energy metabolism pathway, which is independent of oxygen concentration but results in a massive accumulation of lactate in the tumor microenvironment, causing a notable decrease in extracellular matrix pH. This phenomenon, widely known as the "Warburg effect," not only meets the urgent energy demands of rapidly proliferating cancer cells but also facilitates the synthesis of other crucial biomolecules.
To fully exploit the acidic characteristics of the tumor microenvironment, pH-responsive carriers incorporate ionizable weak acid or weak base polymers into their design. These polymers undergo protonation and deprotonation processes, exhibiting selective solubility changes in aqueous environments, thereby precisely controlling drug release towards tumor tissues. Examples of commonly used materials for constructing pH-responsive carriers include weak base polymers such as acrylic acid (AAc), methacrylic acid (MAAc), maleic anhydride (MA) and their derivatives, as well as poly(amidoamine) (PAA or PAMAM). Additionally, poly(β-amino ester) (PbAE) is also widely applied in pH-responsive delivery systems due to its strong pH-dependent solubility.
Specifically, the poly(ethylene oxide)-poly(β-amino ester) (PEO-PbAE) copolymer system, validated through comprehensive in vitro and in vivo studies, has demonstrated high apoptosis rates in MDA-MB-231 breast cancer cells and effective accumulation in SKOV3 human ovarian cancer xenograft models, further underscoring its potential as a drug delivery platform.
It is noteworthy that pH-responsive carriers are not limited to the delivery of small molecule drugs but are also extensively used for the efficient transport of biologics such as genes, siRNA, miRNA, peptides, and proteins. This underscores the flexibility in design and functional diversity of such delivery systems, opening up broad prospects for the development of precision medicine.
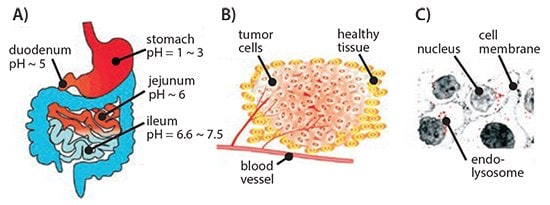
Figure 1. Schematic representation of different pH environments at the organ (A), tissue (B), and cellular (C) levels, which can be exploited for the development of pH-responsive drug delivery systems.
Redox-Responsive System
Tumor tissues exhibit unique redox properties characterized by an oxidative extracellular environment and a reducing intracellular state. This disparity creates a redox potential that serves as a pivotal factor driving the development of redox-responsive delivery vehicles. Compared to the lower glutathione concentrations (2-20 μM) found in the extracellular matrix, the concentrations within the cytoplasm and nucleus significantly increase to 2-10 mM, creating a concentration gradient that poses challenges to the structural design of redox-responsive systems. These systems must maintain stability in highly reducing environments while swiftly responding to specific conditions to release drugs.
Given this background, disulfide bonds (S-S), the most extensively studied redox-sensitive linkage, are widely employed in constructing delivery systems based on polymers, lipids, or proteins. For instance, a series of copolymers with cleavable shells, such as PEG-SS-poly(ε-caprolactone) (PEG-S-S-PCL), PCL-SS-poly(ethyl ethylene phosphate) (PCL-S-S-PEEP), SS-PAA-g-PEG, and dextran-SS-PCL, have been successfully developed as redox-responsive delivery systems with accelerated drug release properties. These systems have not only demonstrated favorable payload activity in vitro but also significantly inhibited tumor growth in vivo, validating their superiority.
Our research team has focused on the development of thiolated gelatin-based protein nanoparticle systems, which have exhibited efficient targeted delivery capabilities to pancreatic cancer cells, both for small molecule drugs and gene therapeutic agents, in models bearing pancreatic human adenocarcinoma xenografts. Additionally, another study loaded these nanoparticles with plasmid vascular endothelial growth factor (VEGF-1), achieving tumor growth inhibition and reduced angiogenesis in an orthotopic MDA-MB-231 human breast cancer model, further expanding their application potential.
Concurrently, we have also explored the Layer-by-Layer (LbL) assembly technique, utilizing poly(vinylpyrrolidone) (PVP)-coated silica nanoparticles as sacrificial templates to construct disulfide-crosslinked poly(methacrylic acid) (PMA) capsules. This innovative design not only offers a novel route for the delivery of proteins and peptides but also showcases broad application prospects as carriers for vaccines or small molecule anticancer drugs.
Enzyme-Responsive System
Proteases, an integral part of tumor physiology, include cancer-associated proteases (CAPs) such as matrix metalloproteinases (MMPs), cathepsins, and urokinase plasminogen activator (uPA), which profoundly influence tumor tissue remodeling and disease progression, invasion, and metastasis. Notably, MMPs, which are overexpressed in most cancer types, are widely recognized as key drivers of cancer development and aggressiveness. Based on this, researchers have designed innovative enzyme-responsive carriers equipped with enzyme-specific peptide sequences that degrade in response to specific proteases in the tumor microenvironment, triggering precise drug release.
One example involves the ingenious combination of protease-sensitive matrices with PEG-diacrylate hydrogel wafers, where the chemotherapeutic drug cisplatin is attached to the MMP-2 or MMP-9 cleavable peptide sequence CGLDD, enabling localized, on-demand drug release. Similarly, dextran-PVGLIG-methotrexate conjugates also exhibit MMP-triggered drug release characteristics and demonstrate antitumor effects in vivo.
Furthermore, the design of non-elastic liposomes has incorporated enzyme-responsive elements, such as the integration of lipid components like DOPE, N-Ac-AA, DOTAP, and PE with protease-responsive linkers. These liposomes transform into fusion proteins upon encountering elastase or proteinase K, enhancing intracellular uptake efficiency. Additionally, the introduction of galactose-modified liposomes combined with a PEG shielding strategy achieves targeted drug delivery to hepatocytes, with MMP-2 serving as the "switch" to activate targeted uptake.
The latest research integrates cholesterol-anchored graft polymers containing the uPA consensus sequence GSGRSAGK and acrylic acid into specific liposome formulations (e.g., mixtures of DOPC, DPPC, and cholesterol). This not only enhances liposome stability but also enables rapid degradation and drug release under enzymatic action. While these research achievements have yet to reach clinical application, they demonstrate immense potential in improving drug efficacy and overcoming low bioavailability, providing valuable insights and directions for future cancer treatment strategies.
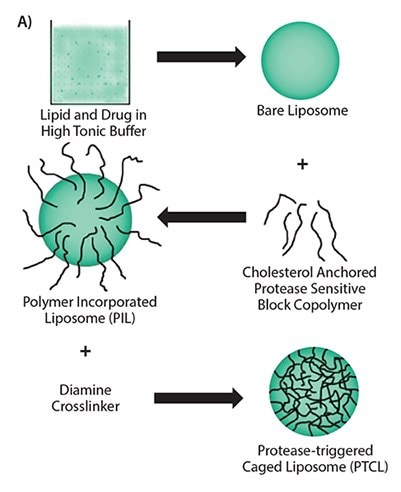
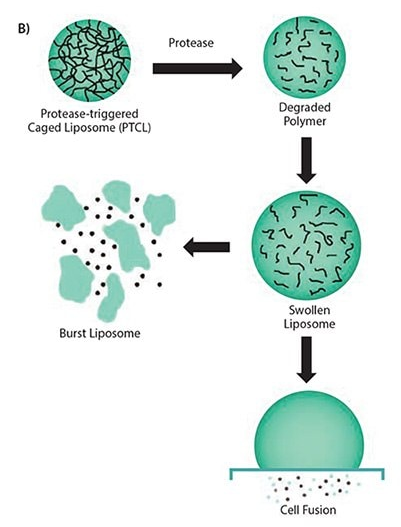
Figure 2. A) Multiple steps involved in the synthesis scheme of uPA-sensitive polymeric caged liposomes. B) Proposed mechanism of the behavior of polymeric caged liposomes in response to the presence of enzymes.
External Regulation System
The external regulation system, also known as an open-loop system, represents an innovative drug delivery platform that distinguishes itself by its ability to precisely manipulate the drug release process in response to specific stimuli from the external environment. This system empowers researchers with unprecedented capabilities to meticulously sculpt the drug release profile of nanocarriers in both temporal and spatial dimensions by finely adjusting the duration and intensity of external stimuli, ensuring that the drug is delivered to the target site accurately, at the desired dose, and when needed.
Within this field, researchers have extensively explored various external stimuli, including thermal, light, acoustic, magnetic, and electrical stimulation, each of which exhibits unique advantages and application prospects:
Thermal Stimulation: Utilizing temperature changes as the triggering mechanism, this method regulates drug release by heating or cooling the carrier, making it suitable for local hyperthermia or scenarios requiring temperature-sensitive release.
Light Stimulation: Leveraging specific wavelengths of light to activate photosensitive molecules within the carrier, this approach enables non-invasive, remote, and precise control over drug release, particularly advantageous for treatments requiring precise spatial localization.
Acoustic Stimulation: Employing ultrasonic waves or other acoustic signals as regulatory means, this method influences the carrier structure through mechanical vibration or cavitation effects, facilitating drug release. It boasts strong penetration capabilities and high safety.
Magnetic Stimulation: By embedding magnetic nanoparticles within the carrier, this technique harnesses external magnetic fields to guide carrier positioning and control drug release, offering possibilities for targeted delivery and dynamic regulation.
Electrical Stimulation: Directing electric fields or currents to act on the carrier, this method triggers drug release through electrochemical reactions or electro-induced deformation, suitable for scenarios requiring rapid response or deep tissue treatment.
These external stimuli not only enrich the toolbox of drug delivery systems but also lay a solid foundation for the realization of advanced medical concepts such as personalized medicine and precision therapy.
Thermoresponsive System
Thermoresponsive materials exhibit a unique property of undergoing significant phase transitions upon reaching specific temperature thresholds, which are marked as the Lower Critical Solution Temperature (LCST) and Upper Critical Solution Temperature (UCST). Below the LCST or above the UCST, these materials remain insoluble; however, upon crossing their respective critical temperatures, they fully transition into a soluble state. Poly(N-isopropylacrylamide) (PNIPAAm), a representative of such thermosensitive materials, exhibits hydrophobic properties in water close to physiological conditions (around 32°C), limiting its ideality for direct application in drug delivery.
Nevertheless, researchers have successfully tailored the phase transition temperature of PNIPAAm to cater to diverse needs in the biomedical field through innovative strategies such as adjusting its side chain structure, polymer molecular weight, overall architecture, or copolymerizing it with other hydrophilic or hydrophobic polymers. Consequently, the research on PNIPAAm derivatives as temperature-sensitive drug carriers has flourished, with widespread applications in micelles, liposomes, hydrogels and nanogels, polymeric vesicles, interpenetrating network structures, membrane materials, and surface modifications of inorganic nanoparticles.
In particular, copolymers of PNIPAAm with materials like poly(N,N-diethylacrylamide) (PDEAAm), poly(N-vinylcaprolactam) (PVCL), PLGA, poly(2-(dimethylamino)ethyl methacrylate), PEG, gelatin, and chitosan have shown immense potential in the delivery of chemotherapeutic drugs and biologics.
Moreover, the development and application of thermosensitive materials extend far beyond drug delivery, with extensive exploration in tissue engineering and regenerative medicine, as well as surface coatings and scaffold materials for bioimaging and diagnostic technologies, opening new avenues for advancements in medical science.
Light-Responsive System
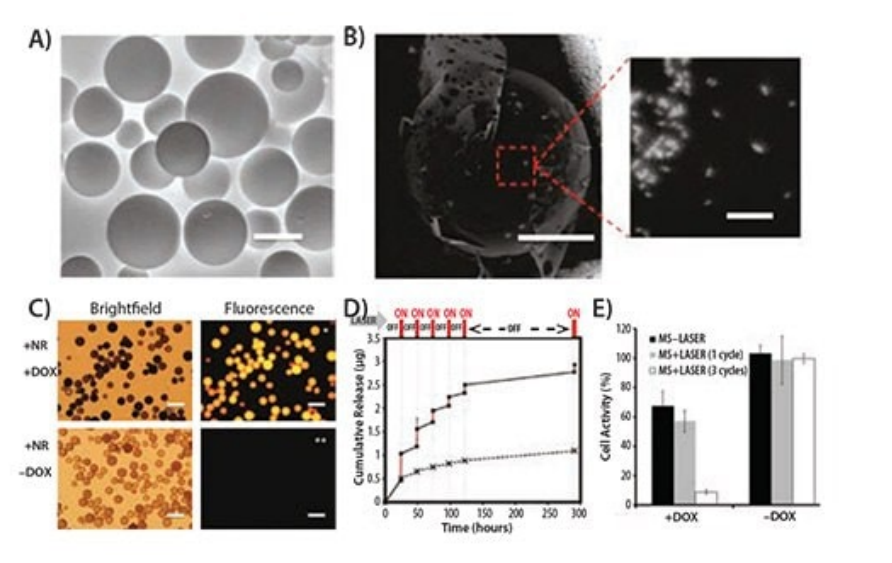
Given its precise controllability in intensity and penetration depth, light has emerged as a popular external stimulus and holds a significant position in drug delivery systems. Following this trend, the application of photosensitive materials in drug carrier design has become increasingly widespread. Among the myriad of light-responsive molecules, azobenzene, o-nitrobenzene, coumarin, and pyrene derivatives are frequently chosen for such designs due to their unique properties. For instance, Jiang et al. innovatively constructed a diblock copolymer based on polyethylene oxide (PEO) and poly(methacrylate) containing pyrene side chains, forming a UV-light-responsive micellar system. Using Nile Red dye as a model drug, they successfully demonstrated the close correlation between drug release efficiency and irradiation time as well as illumination power within this system. However, considering the potential hazards of UV light to organisms, particularly upon prolonged exposure, the scientific community is actively seeking alternative materials sensitive to visible or near-infrared (NIR) light.
In this context, inorganic nanomaterials, particularly anisotropic noble metal nanoparticles with exceptional NIR absorption capabilities, have demonstrated immense potential for application and are being utilized as light absorbers to enhance drug delivery efficiency. Kang et al.'s research serves as a prime example, where they utilized silicon-gold-coated nanorods with strong absorption peaks near 850 nm (Figure 3A-C) and prepared an NIR-responsive drug carrier by polymerizing and crosslinking acrylamide on their surfaces. Experimental results showed that these doxorubicin (DOX)-loaded particles exhibited enhanced cytotoxicity upon 808 nm NIR light irradiation, while toxicity was significantly reduced in the absence of light or in the presence of light without nanoparticles, firmly validating the effectiveness of the light-mediated drug release mechanism.
Hribar et al. further extended this concept by developing a polymer-gold nanorod composite material capable of precise controlled release of small molecule drugs (molecular weight < 800 Da) under NIR light irradiation. They formed a polymer matrix by copolymerizing specific ratios of PbAE macromonomer (A6), tert-butyl acrylate (tBA) monomer, and 2-hydroxyethyl acrylate monomer, and embedded high concentrations of gold nanorods and photoresponsive materials within it. Experimental results indicated that this composite material significantly promoted the in vitro release of DOX upon NIR light irradiation (Figure 3D), subsequently enhancing its toxicity in T6-17 cells (Figure 3E), once again verifying the feasibility and efficiency of the light-controlled release strategy.
Figure 3. A) Environmental scanning electron micrograph of polymer microspheres (MS) made from A6:HEA:tBA (10:20:70). Scale bar is 50 μm. B) Backscattered electron micrograph of microspheres with embedded gold nanorods (bright spots, scale bar = 20 μm), with an enlarged image of the highlighted area showing the nanorods within the microsphere (scale bar = 200 nm). C) Bright-field and fluorescence images of microspheres. Microspheres loaded with DOX exhibit intense fluorescence (top right), while microspheres without DOX show no background fluorescence (bottom right). Scale bars in all images are 100 μm. D) Cumulative drug release profile from microspheres as a function of laser pulses (wavelength = 808 nm) at physiological temperature (37°C). E) Bar chart of T6-17 cell viability after 1 and 3 cycles (laser power = 1.1 W, 5 minutes per cycle) of laser irradiation for MS and DOX-loaded MS.
Ultrasound-responsive System
Ultrasound energy, akin to light intensity, possesses a high degree of customizability, enabling precise adjustment of its intensity and focusing on specific small regions within the body. This characteristic significantly enhances drug release efficiency, hence its widespread designation as High-Intensity Focused Ultrasound (HIFU). Given the extensive application of ultrasound in clinical imaging and diagnosis, ultrasound-based on-demand drug delivery carriers naturally emerge as an ideal choice for constructing "smart" delivery systems.
Ultrasound energy primarily facilitates drug release from carriers through three core mechanisms: firstly, the thermal effect, which leverages the heat generated by ultrasound energy to trigger drug release; secondly, acoustic cavitation, which disrupts carrier structures and promotes drug release by inducing microbubble explosions in liquids via ultrasound; lastly, acoustic radiation force, which directly acts on carriers utilizing the radiation pressure of ultrasound waves, influencing the drug release process. The effective utilization of these mechanisms heavily relies on the duration of ultrasound application and specific parameter settings.
It is noteworthy that the fundamental principles of ultrasound delivery are closely intertwined with thermal regulation, leading to numerous similarities in the design of ultrasound nanoparticle delivery systems with thermally responsive systems. Furthermore, microbubble technology, initially developed to enhance ultrasound imaging clarity, has been ingeniously applied in acoustic cavitation processes to alter cell membrane permeability and serve as direct drug delivery carriers.
The research by Ibsen et al. further showcases the potential of ultrasound technology in drug delivery. Their developed nested liposome system, by generating microbubbles internally to enhance ultrasound responsiveness, successfully achieved efficient delivery of small and large molecules. Similarly, microbubbles loaded with mRNA-lipid complexes, serving as vaccine carriers, successfully achieved target gene transfection and expression in dendritic cells, subsequently promoting changes in cell maturity and effectively inducing T-cell immune responses. These findings strongly demonstrate the innovative and promising nature of ultrasound-assisted delivery as a method, exhibiting immense potential in the biomedical field.
Magnetic Regulation System
Similar to thermally and ultrasonically responsive carriers, magnetically responsive delivery systems rely on external stimuli—in this case, magnetic fields rather than direct heat induction—to trigger the release of payloads. However, by applying an external magnetic field, these carriers can be precisely guided and concentrated in specific regions within the body, adding a unique advantage to the delivery system. Traditionally, magnetic nanomaterials such as superparamagnetic iron oxide nanoparticles (SPIONs) are integrated into polymer, lipid, or protein delivery systems, endowing them with magnetic properties. Notably, SPIONs are also widely used as contrast agents for magnetic resonance imaging (MRI), and when incorporated into delivery vehicles, they can simultaneously provide imaging capabilities with stimulus responsiveness.
When thermally responsive polymers like PNIPAAm are used to coat SPIONs loaded with doxorubicin (DOX), the system exhibits rapid drug release characteristics in a high-temperature environment induced by a magnetic field, once the temperature exceeds the lower critical solution temperature (LCST). Below the LCST, it maintains a slow and sustained release mode. This feature was validated in an in vivo experiment using Buffalo rats with hepatocellular carcinoma, where magnetic guidance significantly increased drug accumulation at tumor sites, improving MRI-based contrast imaging and enhancing therapeutic efficacy.
The research by Majewski et al. further demonstrates the utility of magnetically active carriers as gene delivery tools, particularly their ability to selectively isolate transfected cells from cell populations using the internalization of magnetic nanoparticles. Given the multifunctionality exhibited by magnetically regulated delivery systems, they are now often designed for dual or multiple stimulus-responsive systems to fully leverage the independent response advantages under different stimuli, providing more precise and efficient solutions for drug delivery and therapy.
Electrically Responsive System
A diverse range of materials, both organic and inorganic, have been ingeniously employed in the design of delivery systems that are highly sensitive to external electric fields due to their inherent conductivity. In the field of drug delivery, electrically sensitive materials such as polypyrrole (PPy), ferrocene, and carbon nanotubes have garnered significant attention. In these systems, typically, a weak electric pulse (around 1V) is utilized, which offers numerous advantages over other external stimulation methods: it is easily controllable and applicable, requiring no complex or sophisticated equipment, and can seamlessly integrate into chip-based microdevice designs.
Ge et al. innovatively utilized dodecyltrimethylammonium bromide (DTAB) micelles, supplemented with decanol as a cosurfactant, to successfully polymerize PPy nanoparticles onto a hydrophobic core. Subsequently, these nanoparticles were skillfully embedded within a temperature-sensitive PLGA-PEG-PLGA block copolymer that remains in a solution state at low temperatures (e.g., 4°C) and rapidly transforms into a gel near physiological temperatures (37°C). Furthermore, they loaded daunorubicin and fluorescein into the polymer matrix containing the embedded nanoparticles, resulting in a material that maintains a stable solid hydrogel form at body temperature and exhibits a pronounced response to electric pulses, effectively releasing drugs upon electrical stimulation. When administered subcutaneously to FVB mice in a soluble form, the material rapidly solidified into a gel and successfully released its drug payload within the body under external electric pulses. In contrast, the control group without external stimulation showed lower drug release.
Zhu et al. explored a novel strategy by grafting 4-(3-cyanophenyl)butene (CPB), acting as an electrically responsive "nano-propeller," onto the pore walls of mesoporous silica. Due to its significant intrinsic dipole moment, CPB undergoes rapid reorientation under an electric field, facilitating the swift release of guest molecules (e.g., ibuprofen) from the pores. This discovery not only broadens the application of electric fields in drug delivery but also provides new avenues for precise control over drug release.
Future Perspectives
Currently, the extensive research literature in the field of stimulus-responsive delivery systems underscores their growing importance in medical applications. Nevertheless, it cannot be overlooked that despite the promising prospects, most of these systems remain at the threshold of preclinical research, with only a handful having crossed into clinical trials. Achieving precise control over the linkage between "stimulus" and "response" is one of the key challenges impeding their clinical translation, particularly exacerbated by complex synthetic processes and multifarious component formulations. Most stimulus-responsive delivery systems are still in their nascent stages of development, urgently requiring in-depth optimization of synthesis protocols to ensure their smooth progression into clinical applications.
In the journey from laboratory to clinic, enhancing the accuracy and precision of stimuli becomes an indispensable component. While manipulation of external physical stimuli is relatively straightforward, the unpredictability of internal biological triggering mechanisms adds significant complexities to this process. The marked physiological variations among different patients, organs, and even within the same tumor underscore the heightened demands on the adaptability and flexibility of stimulus-responsive systems. Concurrently, enhancing tissue penetration of external stimuli without damaging surrounding tissues remains a formidable challenge, necessitating continuous optimization of relevant parameters to find the optimal balance.
Moreover, external factors may adversely impact the efficacy of stimulus-responsive delivery systems. For instance, while the EPR (Enhanced Permeability and Retention) effect shows immense potential in facilitating drug accumulation in tumors, its validation in clinical practice remains inadequate. The intricate diversity of the disease microenvironment, encompassing intricate interactions among diseased cells, stromal tissues, immune cells, and more, poses significant barriers for delivery systems to access target cells. In cancer, the heterogeneity at cellular and physiological levels significantly undermines the ability of delivery systems to reach and act on targets.
Notably, current research heavily focuses on in vitro experiments, with relatively scarce explorations into in vivo applications, representing a critical area for future reinforcement. To propel the clinical application of stimulus-responsive delivery systems, our aim is to devise systems that are both simplified and endowed with highly efficient stimulus-responsive characteristics. Despite numerous challenges ahead, the immense potential and advantages exhibited by stimulus-responsive systems undoubtedly lay a solid foundation for them to serve as potent supplements or even alternatives to traditional delivery methods.
References
1. Iyer AK, Singh A, Ganta S, Amiji MM. 2013. Role of integrated cancer nanomedicine in overcoming drug resistance. Advanced Drug Delivery Reviews. 65(13-14):1784-1802. https://doi.org/10.1016/j.addr.2013.07.012
2. Meacham CE, Morrison SJ. 2013. Tumour heterogeneity and cancer cell plasticity. Nature. 501(7467):328-337. https://doi.org/10.1038/nature12624
3. Ganta S, Devalapally H, Shahiwala A, Amiji M. 2008. A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of Controlled Release. 126(3):187-204. https://doi.org/10.1016/j.jconrel.2007.12.017
4. Talekar M, Boreddy SR, Singh A, Amiji M. 2014. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opinion on Biological Therapy. 14(8):1145-1159. https://doi.org/10.1517/14712598.2014.912270
5. Devalapally H, Shenoy D, Little S, Langer R, Amiji M. 2007. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 3. Therapeutic efficacy and safety studies in ovarian cancer xenograft model. Cancer Chemother Pharmacol. 59(4):477-484. https://doi.org/10.1007/s00280-006-0287-5
6. Shenoy D, Little S, Langer R, Amiji M. 2005. Poly(Ethylene Oxide)-Modified Poly(?-Amino Ester) Nanoparticles as a pH-Sensitive System for Tumor-Targeted Delivery of Hydrophobic Drugs: Part 2. In Vivo Distribution and Tumor Localization Studies. Pharm Res. 22(12):2107-2114. https://doi.org/10.1007/s11095-005-8343-0
7. Shenoy D, Little S, Langer R, Amiji M. 2005. Poly(ethylene oxide)-Modified Poly(?-amino ester) Nanoparticles as a pH-Sensitive System for Tumor-Targeted Delivery of Hydrophobic Drugs. 1. In Vitro Evaluations. Mol. Pharmaceutics. 2(5):357-366. https://doi.org/10.1021/mp0500420
8. Gao W, Chan JM, Farokhzad OC. 2010. pH-Responsive Nanoparticles for Drug Delivery. Mol. Pharmaceutics. 7(6):1913-1920. https://doi.org/10.1021/mp100253e
9. Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, Stayton PS. 2010. pH-Responsive Polymeric Micelle Carriers for siRNA Drugs. Biomacromolecules. 11(11):2904-2911. https://doi.org/10.1021/bm100652w
10. Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. 2011. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. Journal of Controlled Release. 152(1):2-12. https://doi.org/10.1016/j.jconrel.2011.01.030
11. Xu J, Singh A, Amiji MM. 2014. Redox-responsive targeted gelatin nanoparticles for delivery of combination wt-p53 expressing plasmid DNA and gemcitabine in the treatment of pancreatic cancer. BMC Cancer. 14(1): https://doi.org/10.1186/1471-2407-14-75
12. Kommareddy S, Amiji M. 2007. Antiangiogenic gene therapy with systemically administered sFlt-1 plasmid DNA in engineered gelatin-based nanovectors. Cancer Gene Ther. 14(5):488-498. https://doi.org/10.1038/sj.cgt.7701041
13. Egeblad M, Werb Z. 2002. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2(3):161-174. https://doi.org/10.1038/nrc745
14. Zhang X, Eden HS, Chen X. 2012. Peptides in cancer nanomedicine: Drug carriers, targeting ligands and protease substrates. Journal of Controlled Release. 159(1):2-13. https://doi.org/10.1016/j.jconrel.2011.10.023
15. Basel MT, Shrestha TB, Troyer DL, Bossmann SH. 2011. Protease-Sensitive, Polymer-Caged Liposomes: A Method for Making Highly Targeted Liposomes Using Triggered Release. ACS Nano. 5(3):2162-2175. https://doi.org/10.1021/nn103362n
16. SCHMALJOHANN D. 2006. Thermo- and pH-responsive polymers in drug delivery?. Advanced Drug Delivery Reviews. 58(15):1655-1670. https://doi.org/10.1016/j.addr.2006.09.020
17. Jiang J, Tong X, Zhao Y. 2005. A New Design for Light-Breakable Polymer Micelles. J. Am. Chem. Soc.. 127(23):8290-8291. https://doi.org/10.1021/ja0521019
18. Kang H, Trondoli AC, Zhu G, Chen Y, Chang Y, Liu H, Huang Y, Zhang X, Tan W. 2011. Near-Infrared Light-Responsive Core?Shell Nanogels for Targeted Drug Delivery. ACS Nano. 5(6):5094-5099. https://doi.org/10.1021/nn201171r
19. Hribar KC, Lee MH, Lee D, Burdick JA. 2011. Enhanced Release of Small Molecules from Near-Infrared Light Responsive Polymer?Nanorod Composites. ACS Nano. 5(4):2948-2956. https://doi.org/10.1021/nn103575a
20. Hernot S, Klibanov AL. 2008. Microbubbles in ultrasound-triggered drug and gene delivery. Advanced Drug Delivery Reviews. 60(10):1153-1166. https://doi.org/10.1016/j.addr.2008.03.005
21. DeTemmerman M, Dewitte H, Vandenbroucke RE, Lucas B, Libert C, Demeester J, De Smedt SC, Lentacker I, Rejman J. 2011. mRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials. 32(34):9128-9135. https://doi.org/10.1016/j.biomaterials.2011.08.024
22. Purushotham S, Chang PEJ, Rumpel H, Kee IHC, Ng RTH, Chow PKH, Tan CK, Ramanujan RV. 2009. Thermoresponsive core?shell magnetic nanoparticles for combined modalities of cancer therapy. Nanotechnology. 20(30):305101. https://doi.org/10.1088/0957-4484/20/30/305101
23. Majewski AP, Schallon A, Jérôme V, Freitag R, Müller AHE,Schmalz H. 2012. Dual-Responsive Magnetic Core?Shell Nanoparticles for Nonviral Gene Delivery and Cell Separation. Biomacromolecules. 13(3):857-866. https://doi.org/10.1021/bm2017756
24. Cheng R, Meng F, Deng C, Klok H, Zhong Z. 2013. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 34(14):3647-3657. https://doi.org/10.1016/j.biomaterials.2013.01.084
25. Ge J, Neofytou E, Cahill TJ, Beygui RE, Zare RN. 2012. Drug Release from Electric-Field-Responsive Nanoparticles. ACS Nano. 6(1):227-233. https://doi.org/10.1021/nn203430m
26. Zhu Y, Liu H, Li F, Ruan Q, Wang H, Fujiwara M, Wang L, Lu GQ(. 2010. Dipolar Molecules as Impellers Achieving Electric-Field-Stimulated Release. J. Am. Chem. Soc.. 132(5):1450-1451. https://doi.org/10.1021/ja907560y
27. Mura S, Nicolas J, Couvreur P. 2013. Stimuli-responsive nanocarriers for drug delivery. Nature Mater. 12(11):991-1003. https://doi.org/10.1038/nmat3776
For more information, visit our website: www.aladdinsci.com.