Substituted Azetidines in pharmaceutical chemistry, organic synthesis, and biochemistry

Introduction
Azetidine is a saturated heterocyclic organic compound composed of three carbon atoms and one nitrogen atom. At room temperature, it exists in the form of a liquid with a strong ammonia odor and a strong alkalinity compared to most secondary amines.
![]()
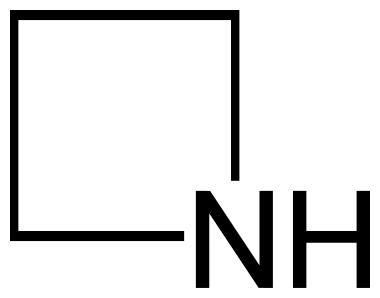
Fig.1 Azetidine
Azetidines and their derivatives rarely appear in the natural molecular structure of nature due to their high rigidity and ring tension. The virtually unexplored four-membered heterocycles, azetidines, are a special class of saturated azetidines present in the family of saturated azetidines. Although the synthesis of substituted azetidines is often very challenging, they continue to be of great interest to chemists due to their im portant role in catalysis, stereoselective synthesis and medicinal chemistry. In recent years, it has become increasingly important to efficiently obtain substituted heterocyclic derivatives due to the increasing need to improve the saturation and three-dimensionality of drug candidate molecules.[1]
Synthesis and Occurrence
Azetidine can be produced by reducing 2-azetidinones (β-lactams) with lithium aluminum hydride. A more efficient method is to use a mixture of lithium aluminium hydride and aluminum trichloride, which is the source of "AlClH2" and "AlCl2H".[2] Azetidine can also be made from 3-amino-1-propanol by a multi-step reaction. [3]
The regioselective and diastereoselective synthesis of 2-arylazetidines can be carried out via cycloaddition of suitably substituted ethylene oxide. It follows Baldwin's rules and has remarkable functional group tolerance.
Azetidine and their derivatives are relatively rare chemical skeletons in natural products. They are one of the constituents of mugenic acid and Penaresidin. Azetidine-2-carboxylic acid, the most abundant azetidine-containing natural product, is a non-proteinogenic amino acid homologue of proline, which can be incorrectly incorporated into proteins to replace proline. [4]
Application
Pharmaceutical Chemistry:
In the last decade, fragment-based drug design (FBDD) has emerged as a highly promising approach for the development of new drugs. Since most organic small molecules lack inherent molecular rigidity, this seems to be a serious obstacle to this promising approach (Fig.2). Conformationally constrained rigid molecules show higher reproducibility of results when utilizing in silico screening methods. Altering the conformational flexibility of a molecule by introducing a ring or ring system is one of the most commonly used methods. Small monocyclic backbones are preferred over other conformationally limited backbones due to their lesser effect on the total molecular weight and lipophilicity of the molecule. In contrast, azetidines are the smallest nitrogen-containing saturated heterocycles with reasonable chemical stability. Azetidines-containing molecular blocks have been widely used in drug design for some time; one of the best-known and successful examples is the hypertension treatment Azelnidipine, a calcium channel blocker.
![]()
Fig.2 Noteworthy examples of biologically active drug leads and natural products incorporating the azedidine nucleus
Organic Synthesis:
The limited availability of azetidines relative to other azetidines such as azetidines, pyrrolidines or piperidines has resulted in much less research and progress on them. Recently chemists have developed synthetic routes for strained heterocycles made from readily available enantiomerically pure starting compounds as feedstock. Upon ring strain, they become excellent candidates for nucleophilic ring opening or ring expansion reactions to synthesize higher ring systems or highly substituted acyclic amines.
Biologic Chemistry:
Novel amino acid syntheses have received much attention, where the difficulty lies in maintaining the nitrogen heterocycles in the amino acid portion, as also shown by many synthetic methods for proline, piperidinic acid and glutamic acid analogs. On the contrary, the synthesis of four-membered heterocyclic amino acids has been rarely reported, probably due to the lack of efficient synthetic methods for the preparation of functionalized azetidines, especially in the enantiomerically pure form.A new route to synthesize azetidine amino acids with a general structure has been reported by Rabassoa's group and is shown in Fig. 3. The nature of the R group located at C-4 therein is derived from the enantiomerically pure β-amino alcohol.[5]
Fig.3 The examples of azetidinic amino acids
Reference
1. Recent advances in the Chemistry of Metallated Azetidines, J. Name., 2013, 00, 1-3 https://doi.org/10.1039/C6OB01665K
2. Alcaide, Benito; Almendros, Pedro; Aragoncillo, Cristina (2007). "Β-Lactams: Versatile Building Blocks for the Stereoselective Synthesis of Non-β-Lactam Products". Chemical Reviews. 107 (11): 4437–4492. https://doi.org/10.1021/cr0307300
3. Donald H. Wadsworth (1973). "Azetidine". Organic Syntheses. 53: 13. https://doi.org/10.15227/orgsyn.053.0013
4. Kovács, Ervin; Ferenc, Faigl; Zoltan, Mucsi (Aug 10, 2020). "Regio- and Diastereoselective Synthesis of 2-Arylazetidines. Quantum Chemical Explanation of Baldwin's Rules for the Ring-formation Reactions of Oxiranes". Journal of Organic Chemistry. 85 (17): 11226–11239. https://doi.org/10.1021/acs.joc.0c01310
Azetidinic amino acids: stereocontrolled synthesis and pharmacological characterization as ligands for glutamate receptors and transporters, Org. Biomol. Chem. , 2005, 3 , 3926–3936 https://doi.org/10.1039/B509514J


