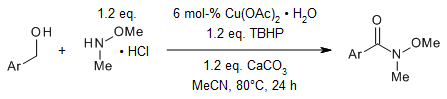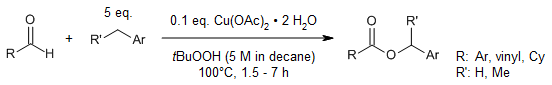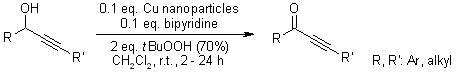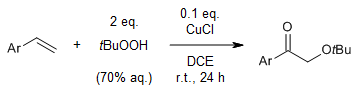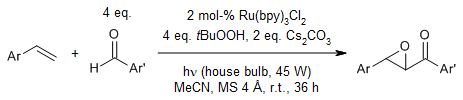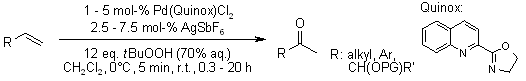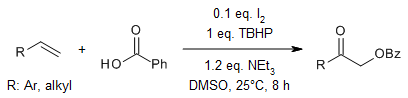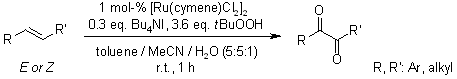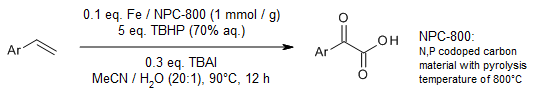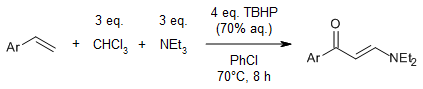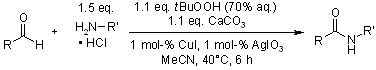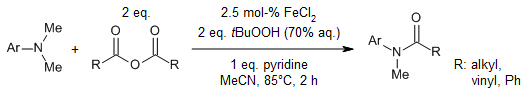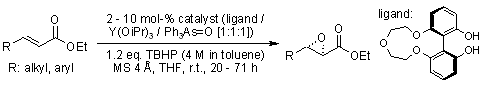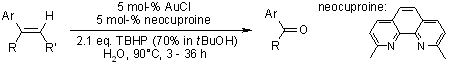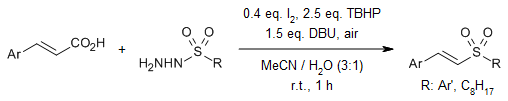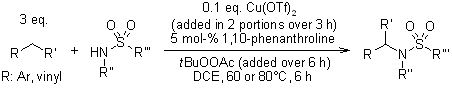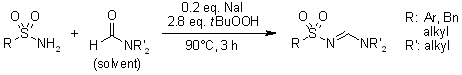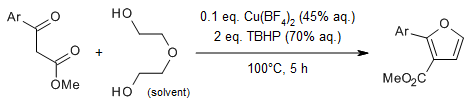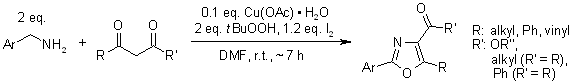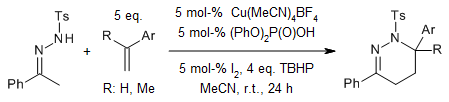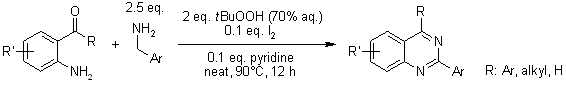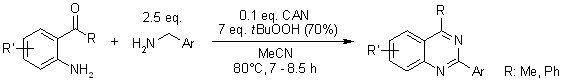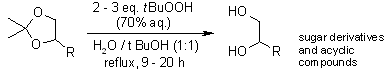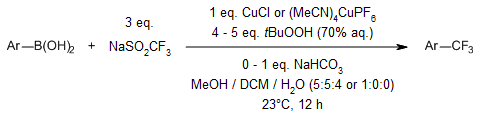tert-Butyl hydroperoxide, TBHP

Product Manager:Nick Wilde
Name Reactions

Sharpless Epoxidation
Recent Literature

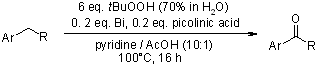
Various aromatic, aliphatic and conjugated alcohols were transformed into the corresponding carboxylic acids and ketones in good yields with aq 70% t-BuOOH in the presence of catalytic amounts of bismuth(III) oxide. This method possesses does not involve cumbersome work-up, exhibits chemoselectivity and proceeds under ambient conditions. The overall method is green.
P. Malik, D. Chakraborty, Synthesis, 2010, 3736-3740.
https://doi.org/10.1055/s-0030-1258221
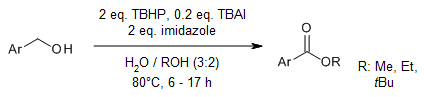
The use of tert-butyl hydroperoxide (TBHP) as a terminal oxidant in the presence of catalytic amount of tetrabutylammonium iodide and imidazole enables a transition-metal-free synthesis of aryl esters in high yield starting from benzylic primary alcohols and aliphatic alcohols. These reactions are highly chemoselective and tolerate a wide range of substituents.
S. Nandy, A. Ghatak, A. K. Das, S. Bhar, Synlett, 2018, 29, 2208-2212.
https://doi.org/10.1055/s-0037-1610247
A highly effective synthesis of methyl esters from benzylic alcohols, aldehydes, or acids via copper-catalyzed C-C cleavage from tert-butyl hydroperoxide is easily accessible and practical and offers an alternative to the traditional way.
Y. Zhu, H. Yan, L. Lu, D. Liu, G. Rong, J. Mao, J. Org. Chem., 2013, 78, 9898-9905.
https://doi.org/10.1021/jo4016387
A highly effective synthesis of methyl esters from benzylic alcohols, aldehydes, or acids via copper-catalyzed C-C cleavage from tert-butyl hydroperoxide is easily accessible and practical and offers an alternative to the traditional way.
Y. Zhu, H. Yan, L. Lu, D. Liu, G. Rong, J. Mao, J. Org. Chem., 2013, 78, 9898-9905.
https://doi.org/10.1021/jo4016387
A copper-catalyzed oxidative cleavage reaction of terminal and internal alkynes using NFSI and TBHP provides aryl ketone products in good yields. NFSI not only functioned as N-centered radical precursor but also engaged in the aryl group migration. Mechanistic studies also suggested the important role of water.
L. Tang, F. Yang, H. Cheng, C. Tan, C. Jin, H. Chen, Y. Huang, S. Zhang, S. Zhang, W. Song, J. Tan, Org. Lett., 2020, 22, 8618-8623.
https://doi.org/10.1021/acs.orglett.0c03201
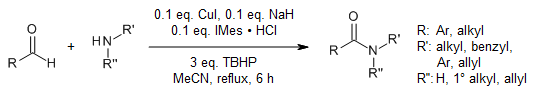
The combination of copper(I) iodide and an N-heterocyclic carbene catalyzes an oxidative amidation of aldehydes with amines in the presence of tert-butyl hydroperoxide. The method is simple and practicable, has a broad substrate scope, and uses economical, feasible, and abundant reagents.
A. Singh, A. K. Narula, Synlett, 2021, 32, 718-722.
https://doi.org/10.1055/a-1343-5203
The use of tert-butyl hydroperoxide as an oxidant and an inexpensive and air stable copper catalyst enables a simple and efficient protocol for the oxidative amidation of commercially affordable alcohols to Weinreb amides in very good yields. The reaction tolerates various functional groups.
S. L. Yedage, B. M. Bhanage, Synthesis, 2015, 47, 526-532.
https://doi.org/10.1055/s-0034-1379583
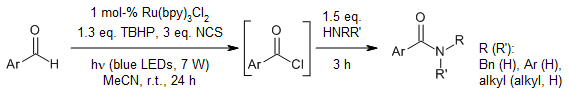
Using an efficient visible-light photocatalysis-based method, a mixture of an aldehyde, tert-butyl hydrogen peroxide, and N-chlorosuccinimide afforded an acid chloride in the presence of Ru(bpy)3Cl2 as photocatalyst. A subsequent reaction with an amine provided the corresponding amide.
N. Iqbal, E. J. Cho, J. Org. Chem., 2016, 81, 1905-1911.
https://doi.org/10.1021/acs.joc.5b02726
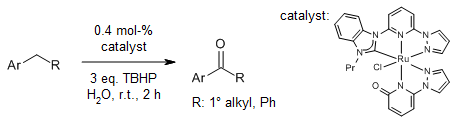
Ru complexes with tridentate hybrid ligands showed a very strong catalytic activity in water at room temperature for benzylic C-H oxidation. Whereas NHCs with a stronger donor ability stabilize the Ru center; nitrogen ligands with a relatively weaker donor ability release from the Ru center, so that they induce a reaction.
C.-B. Bo, Q. Bu, X. Li, G. Ma, D. Wei, C. Guo, B. Dai, N. Liu, J. Org. Chem., 2020, 85, 4324-4334.
https://doi.org/10.1021/acs.joc.0c00025
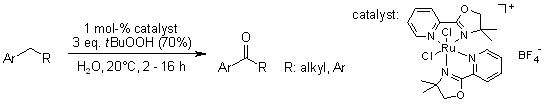
The cationic complex [(pymox-Me2)RuCl2]+BF4- is a highly effective catalyst for the C-H bond oxidation of aryl alkanes in water using tert-butyl hydroperoxide as oxidant to yield various aryl ketones at room temperature in water as solvent. A solvent-caged oxygen rebounding mechanism via a Ru(IV)-oxo intermediate species is suggested.
C. S. Yi, K.-H. Kwon, D. W. Lee, Org. Lett., 2009, 11, 1567-1569.
https://doi.org/10.1021/ol900097y
The bismuth and picolinic acid-catalyzed oxidation of alkyl arenes with tert-butyl hydroperoxide in pyridine and acetic acid gave benzylic ketones in good yields. Alternatively, oxidation of methyl arenes gave the corresponding substituted benzoic acids. A radical mechanism is discussed.
Y. Bonvin, E. Callens, I. Larrosa, D. A. Henderson, J. Oldham, A. J. Burton, A. G. M. Barrett, Org. Lett., 2005, 7, 4549-4552.
https://doi.org/10.1021/ol051765k
Copper(II) catalyzes a cross dehydrogenative coupling (CDC) reaction of aldehydes with alkylbenzenes in the presence of TBHP to yield benzylic esters.
S. K. Rout, S. Guin, K. K. Ghara, A. Banerjee, B. K. Patel, Org. Lett., 2012, 14, 3982-3985.
https://doi.org/10.1021/ol301756y
In an unusual oxidative coupling reaction of isocyanide and toluene derivatives using tetrabutylammonium iodide (TBAI) as a catalyst, the isocyano group acts formally as an N1 synthon, thus expanding the reactivity profile of isocyanides.
Z. Liu, X. Zhang, J. Li, F. Li, C. Li, X. Jia, J. Li, Org. Lett., 2016, 18, 4032-4035.
https://doi.org/10.1021/acs.orglett.6b01928
A metal-free and one-pot two-step synthesis of aryl carboxylic acids from aryl alkyl ketones has been performed with iodine as the catalyst, DMSO and TBHP as the oxidants. Various aryl alkyl ketones could be converted into their corresponding aryl carboxylic acids in very good yields.
L. Xu, S. Wang, B. Chen, M. Li, X. Hu, B. Hu, L. Jin, N. Sun, Z. Shen, Synlett, 2018, 29, 1505-1509.
https://doi.org/10.1055/s-0037-1609751
An efficient, metal-free domino protocol for the synthesis of benzamides from ethylarenes proceeds through the formation of triiodomethyl ketone intermediate in the presence of iodine as the promoter and TBHP as an oxidant followed by nucleophilic substitution with aqueous ammonia. This operationally simple, functional-group-tolerant tandem approach provides an easy access to the broad range of biologically important benzamides.
K. S. Vadagaonkar, H. P. Kalmode, S. Prakash, A. C. Chaskar, Synlett, 2015, 26, 1677-1682.
https://doi.org/10.1055/s-0034-1380210
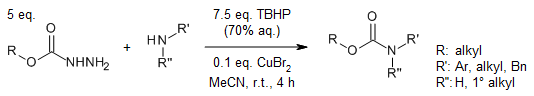
A copper-catalyzed cross-coupling reaction of amines with alkoxycarbonyl radicals generated from carbazates provides carbamates under mild conditions. This environmentally friendly approach is compatible with a wide range of amines, including aromatic/aliphatic and primary/secondary substrates.
S.-N. Wang, G.-Y. Zhang, A. Shoberu, J.-P. Zou, J. Org. Chem., 2021, 86, 9067-9075.
https://doi.org/10.1021/acs.joc.1c01031
A highly efficient oxidation of propargylic alcohols to ynones is catalyzed by copper nanoparticles (Cu Nps) with TBHP or air as oxidants. With bipyridine as the ligand, the reaction was accelerated significantly and led in good to excellent yields to a variety of propargylic alcohols.
C. Han, M. Yu, W. Sun, Y. Yao, Synlett, 2011, 2363-2368.
https://doi.org/10.1055/s-0030-1261227
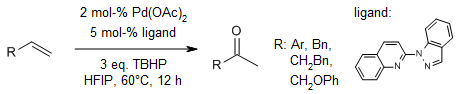
Pd-Catalyzed TBHP-Mediated Selective Wacker-Type Oxidation and Oxo-acyloxylation of Olefins Using a 2-(1H-Indazol-1-yl)quinoline Ligand
S. Zhang, J. Zhang, H. Zou, Org. Lett., 2023, 25, 1850-1855.
https://doi.org/10.1021/acs.orglett.3c00326
A practical and environmentally friendly oxidation of aryl olefins to arylethanone derivatives by using a Cu(I) catalyst and tert-butyl hydroperoxide (TBHP) provides 2-tert-butoxy-1-arylethanones in good yields under mild conditions with high selectivity. In this method, TBHP acts not only as an oxidant but also as the tert-butoxy and carbonyl oxygen sources.
J. Zhang, D. Xiao, H. Tan, W. Liu, J. Org. Chem., 2020, 85, 3929-3935.
https://doi.org/10.1021/acs.joc.9b03156
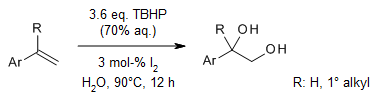
Iodine catalyzes an environment-friendly and efficient dioxygenation of aryl alkenes for the construction of vicinal diols in water as solvent with tert-butylhydroperoxide (TBHP) as the oxidant. The protocol is efficient, sustainable, and operationally simple. In addition, bisperoxides could be obtained in good yields with Na2CO3 as additive and propylene carbonate as solvent.
X. Gao, J. Lin, L. Zhang, X. Luo, G. Guo, N. Peng, H. Xu, Y. Liu, J. Org. Chem., 2021, 86, 15469-15480.
https://doi.org/10.1021/acs.joc.1c01968
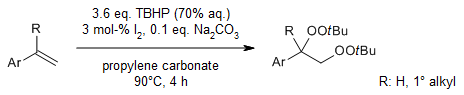
Iodine catalyzes an environment-friendly and efficient dioxygenation of aryl alkenes for the construction of vicinal diols in water as solvent with tert-butylhydroperoxide (TBHP) as the oxidant. The protocol is efficient, sustainable, and operationally simple. In addition, bisperoxides could be obtained in good yields with Na2CO3 as additive and propylene carbonate as solvent.
X. Gao, J. Lin, L. Zhang, X. Luo, G. Guo, N. Peng, H. Xu, Y. Liu, J. Org. Chem., 2021, 86, 15469-15480.
https://doi.org/10.1021/acs.joc.1c01968
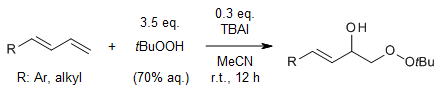
TBAI catalyzes an efficient, mild, and regioselective synthesis of 4-aryl/alkyl-1-peroxy-but-3-en-2-ols in good yields from 1-substituted-1,3-butadienes in the presence of hydroperoxides and water. This regioselective orthogonal dioxygenation of a diene can be executed in a simple operation and tolerates a wide range of substrates.
A. Kumar, G. N. Khatun, R. A. Fernandes, Org. Lett., 2023, 25, 4313-4317.
https://doi.org/10.1021/acs.orglett.3c01393
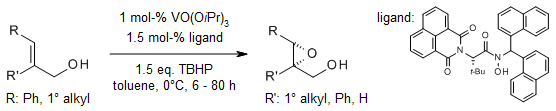
Chiral amino acid-based hydroxamic acids can be effective asymmetric catalysts for the epoxidation of allylic alcohols, especially disubstituted allylic alcohols. The mild reaction conditions, e.g., reasonable temperature, low degree of catalyst loading, and halogen-free solvent, extend the scope of this process.
Y. Hoshino, H. Yamamoto, J. Am. Chem. Soc., 2000, 122, 10452-10453.
https://doi.org/10.1021/ja002602o
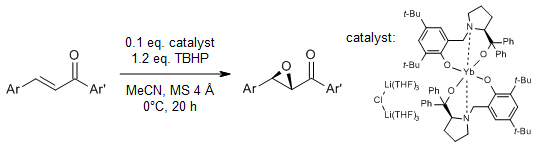
A simple and efficient enantioselective epoxidation of α,β-unsaturated ketones is catalyzed by rare-earth metal amides in the presence of phenoxy-functionalized chiral prolinols at room temperature using tert-butylhydroperoxide (TBHP) as the oxidant. The combination of an Yb-based amide and a chiral proligand provided chiral epoxides in excellent yields and enantiomeric excess of up to 99%.
C. Zeng, D. Yuan, B. Zhao, Y. Yao, Org. Lett., 2015, 17, 2242-2245.
https://doi.org/10.1021/acs.orglett.5b00833
Heterobimetallic complexes stabilized by chiral phenoxy-functionalized prolinolate are highly active in catalyzing the epoxidation of α,β-unsaturated ketones, while the enantioselectivity varies according to the ionic radii of the rare earth center. A series of chalcone derivatives were converted to chiral epoxides in good ee at 0°C using TBHP as the oxidant.
Q. Qian, Y. Tan, B. Zhao, T. Feng, Q. Shen, Y. Yao, Org. Lett., 2014, 16, 4516-4519.
https://doi.org/10.1021/ol5020398
Under the synergistic actions of photocatalyst Ru(bpy)3Cl2, tert-butyl hydroperoxide, cesium carbonate, and visible light irradiation, a range of styrenes and benzaldehydes smoothly form α,β-epoxy ketones via visible-light-enabled photocatalytic generation of acyl radicals as key intermediates.
J. Li, D. Z. Wang, Org. Lett., 2015, 17, 5260-5263.
https://doi.org/10.1021/acs.orglett.5b02629
Utilizing the rapidly synthesized Quinox ligand and commercially available aqueous TBHP, a Wacker-type oxidation efficiently converts even traditionally challenging substrates such as protected allylic alcohols to the corresponding oxidized products. Enantioenriched substrates undergo oxidation with complete retention of enantiomeric excess.
B. W. Michel, A. M. Camelio, C. N. Cornell, M. S. Sigman, J. Am. Chem. Soc., 2009, 131, 6076-6077.
https://doi.org/10.1021/ja901212h
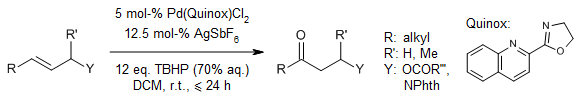
Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP.
R. J. DeLuca, J. L. Edwards, L. D. Steffens, B. W. Michel, X. Qiao, C. Zhu, S. P. Cook, M. S. Sigman, J. Org. Chem., 2013, 78, 1682-1683.
https://doi.org/10.1021/jo302638v
I2-catalyzed oxo-acyloxylation of alkenes and enol ethers with carboxylic acids provides α-acyloxyketones and esters in high yields. This unprecedented regioselective oxidative process employs TBHP and Et3N in stoichiometric amounts under metal-free conditions in DMSO as solvent. α-Acyloxyketones can be converted in situ to monoprotected diol derivatives in excellent yields upon treatment with BH3·SMe2.
R. N. Reddi, P. K. Prasad, A. Sudalai, Org. Lett., 2014, 16, 5674-5677.
https://doi.org/10.1021/ol5027393
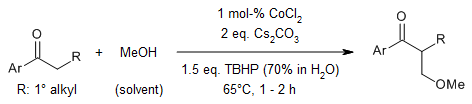
A cobalt-catalyzed α-methoxymethylation of ketones with methanol as a sustainable C1 source, cheap CoCl2·6H2O as catalyst and TBHP as oxidant provides the methoxymethylated products within a short reaction time in very good yield. α-Aminomethylated ketones can be produced by a one-pot methylenation/aza-Michael addition sequence or by a base-mediated conversion of the α-methoxymethyl ketones.
J. Yang, S. Chen, H. Zhou, C. Wu, B. Ma, J. Xiao, Org. Lett., 2018, 20, 6774-6779.
https://doi.org/10.1021/acs.orglett.8b02892
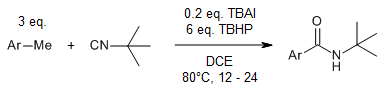
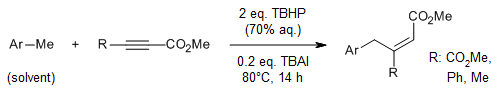
An sp3 C-H bond-transformation reaction of methylarenes provides the corresponding allylbenzene derivatives in good yields in the presence of tetrabutylammonium iodide and tert-butyl hydroperoxide at 80 °C.
F. Shahsavari, A. Abbasi, M. Ghaznafarpour-Darjjani, S. M. Ghafelebashi, M. Daftari-Besheli, Synlett, 2017, 28, 1646-1648.
https://doi.org/10.1055/s-0036-1588793
A ruthenium-catalyzed oxidation of alkenes allows an efficient route to α-diketones using TBHP as an oxidant, is highly functional group tolerant and practically convenient, requires no additional ligand, and operates under mild conditions with short reaction times. Based upon experimental observations, a plausible mechanism is proposed.
S. Chen, Z. Liu, E. Shi, L. Chen, W. Wei, H. Li, Y. Cheng, X. Wan, Org. Lett., 2011, 13, 2274-2277.
https://doi.org/10.1021/ol200716d
A concerted metallophotoredox catalysis enables an efficient decarboxylative functionalization of α,β-unsaturated carboxylic acids with aryl iodides in the presence of perylene bisimide dye to afford 1,2-diketones.
S. Chand, A. K. Pandey, R. Singh, K. N. Singh, J. Org. Chem., 2021, 86, 6343-6350.
https://doi.org/10.1021/acs.joc.1c00322
A recyclable, bifunctional iron nanocomposite catalyzes an efficient synthesis of α-keto acids via oxidation of alkenes using TBHP as oxidant. A variety of alkenes with different functional groups were smoothly oxidized into their corresponding α-keto acids in good yields.
T. Song, Z. Ma, X. Wang, Y. Yang, Org. Lett., 2021, 23, 5917-5921.
https://doi.org/10.1021/acs.orglett.1c02021
A copper-catalyzed one-pot strategy for the synthesis of α-ketoamides from 1-arylethanols is highly efficient and delivers product in very good yields via alcohol oxidation, sp3 C-H oxidation, and oxidative amidation.
N. Sharma, S. S. Kotha, N. Lahiri, G. Sekar, Synthesis, 2015, 47, 726-736.
https://doi.org/10.1055/s-0034-1379975
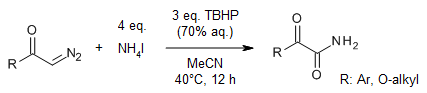
A simple and efficient oxidative coupling of diazoesters and α-diazoketones with NH4I provides primary oxamates and α-ketoamides in good yields. This metal-free protocol is performed under mild conditions and has a wide substrate scope.
H. Wang, Y. Zhao, Y. Zheng, S. Fang, J. Li, X. Wan, J. Org. Chem., 2020, 85, 3050-3058.
https://doi.org/10.1021/acs.joc.9b02952
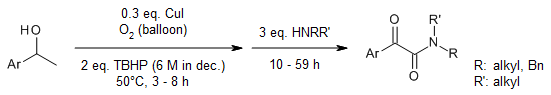
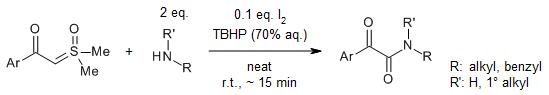
The reaction of sulfoxonium ylides with primary or secondary amines afforded α-ketothioamides in the presence of elemental sulfur, whereas α-ketoamides were produced when I2 and TBHP were present. This simple, scalable reaction proceeded well at room temperature, tolerated a range of functional group, and generated the corresponding products in very good yields.
T. N. Chaubey, P. J. Borpatra, A. Sharma, S. K. Pandey, Org. Lett., 2022, 24, 8062-8066.
https://doi.org/10.1021/acs.orglett.2c03371
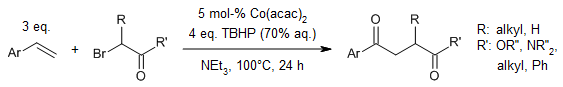
In a Co-catalyzed reaction for the construction of 1,4-dicarbonyls, a cascade organocobalt addition/trapping/Kornblum-DeLaMare rearrangement were involved. The reaction offers easy availability of starting materials, wide substrate scope, high functionality tolerance, and operational simplicity.
F. Zhang, P. Du, J. Chen, H. Wang, Q. Luo, X. Wan, Org. Lett., 2014, 16, 1932-1935.
https://doi.org/10.1021/ol5004687
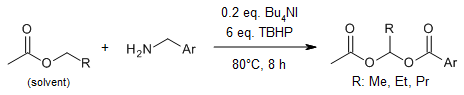
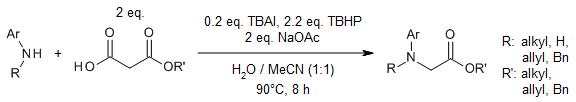
In the presence of TBAI/TBHP, treatment of esters possessing a methylene carbon α-to oxygen with benzylamines provides bis-esters rather than the expected amides. Under these oxidative conditions, benzylamines generate less nucleophilic carboxylates, which couple at sp3 C-H bonds of esters and cyclic ethers to yield bis-acyl ketals and α-acyloxy ethers, respectively.
G. Majji, S. Rajamanickam, N. Khatun, S. K. Santra, B. K. Patel, J. Org. Chem., 2015, 80, 3440-3446.
https://doi.org/10.1021/jo502903d
J. Zhang, P. Zhou, A. Yin, S. Zhang, W. Liu, J. Org. Chem., 2021, 86, 8980-8986.
https://doi.org/10.1021/acs.joc.1c00823
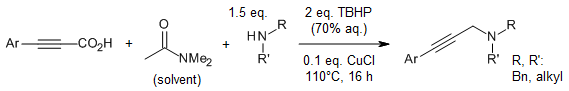
A copper-based system enables methylene insertion between an amine and an alkyne counterpart, via C-N bond cleavage of N,N-dimethylacetamide. The method gives an expedient access to a broad range of propargylic amines in good yields.
A. Rahaman, R. D. Shinde, S. Bhadra, J. Org. Chem., 2023, 88, 1884-1889.
https://doi.org/10.1021/acs.joc.2c02584
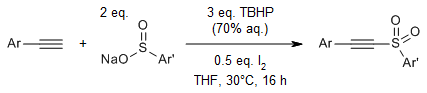
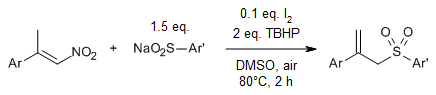
Regiospecific radical reactions of β-alkyl nitroalkenes with sulfonyl hydrazides provide allyl sulfones with high regioselectivity in the presence of dimethylformamide (DMF), whereas reactions in acetonitrile provide vinyl sulfones.
Y. Wang, G. Xiong, C. Zhang, Y. Chen, J. Org. Chem., 2021, 86, 4018-4026.
https://doi.org/10.1021/acs.joc.0c02869
A copper/cobalt-catalyzed oxysulfonylation of alkenes with sulfonylazides and tert-butyl hydroperoxide provides β-ketosulfones and β-sulfonyl peroxides good yields under mild conditions. This methodology applies sulfonylazides as a new sulfonyl radical source and features a wide substrate scope and good functional group tolerance.
R. Chen, Y. Tang, X. He, K.-K. Wang, L. Ding, L. Liu, Org. Lett., 2023, 25, 5454-5458.
https://doi.org/10.1021/acs.orglett.3c01777
A regio- and stereoselective copper-catalyzed sulfonylation of alkynyl imines with sulfonyl hydrazides provides a series of (E)-β-sulfonyl enones in good yields. Mechanistic studies suggest a radical process.
R. Chen, S. Li, J. Zhang, J. Cao, K.-K. Wang, T. Meng, L. Liu, J. Org. Chem., 2022, 87, 13322-13330.
https://doi.org/10.1021/acs.joc.2c01192
A facile photocatalyzed strategy for difunctionalization of styrenes in the presence of CS2 and amines provides β-keto dithiocarbamates. However, 4-nitrostyrene and 2-vinylpyridine can only be converted to 2-arylethylthiocarbamates.
R. K. Vishwakarma, S. Kumar, K. N. Singh, Org. Lett., 2021, 23, 4147-4151.
https://doi.org/10.1021/acs.orglett.1c01059
Olefin substrates can be converted to the corresponding enones or 1,4-enediones in very good yields in short reaction times using a Cu(II) 2-quinoxalinol salen complex as the catalyst and tert-butyl hydroperoxide (TBHP) as the oxidant via allylic activation. The reaction tolerates many additional functional groups.
Y. Li, T. B. Lee, T. Tang, A. V. Gamble, A. E. V. Gorden, J. Org. Chem., 2012, 77, 4628-4633.
https://doi.org/10.1021/jo300372q
Dirhodium(II) caprolactamate effectively catalyzes the allylic oxidation of a variety of olefins and enones with tert-butyl hydroperoxide as terminal oxidant. The reaction is completely selective, tolerant of air and moisture, and can be performed with as little as 0.1 mol % catalyst in minutes.
A. E. Lurain, A. Maestri, A. R. Kelli, P. J. Carroll, P. J. Walsh, J. Am. Chem. Soc., 2004, 126, 13622-13623.
https://doi.org/10.1021/ja045330o
The allylic oxidation of cyclic alkenes with a copper-aluminum mixed oxide as catalyst in the presence of a carboxylic acid and tert-butyl hydroperoxide as the oxidant gives the corresponding allylic esters. When l-proline is employed, the allylic alcohol or ketone is obtained.
A. L. García-Cabeza, R. Marín-Barrios, F. J. Moreno-Dorado, M. J. Ortega, G. M. Massanet, F. M. Guerra, Org. Lett., 2014, 16, 1598-1601
https://doi.org/10.1021/ol500198c
A new and simple method is described for the one-step oxidation of α,β-enones to 1,4-enediones in good yields using t-butylhydroperoxide as stoichiometric oxidant and 20% Pd(OH)2 on carbon as catalyst. The same reagents have been used to convert ethylene ketals of α,β-enones to the corresponding monoethylene ketals of 1,4-enediones. The mechanism is discussed.
J.-Q. Yu, E. J. Corey, J. Am. Chem. Soc., 2003, 125, 3232-3233.
https://doi.org/10.1021/ja0340735
A new catalytic system for the asymmetric epoxidation of allylic alcohols has been developed featuring high enantioselectivity for Z olefins, catalyst loading of less than 1 mol%, reaction temperatures of 0°C to room temperature over a shorter time, use of aqueous tert-butyl hydroperoxide (TBHP) instead of anhydrous TBHP as an achiral oxidant, and simple workup procedures for small expoxy alcohols.
W. Zhang, A. Basak, Y. Kosugi, Y. Hoshino, H. Yamamoto, Angew. Chem. Int. Ed., 2005, 44, 4389-4391.
https://doi.org/10.1002/anie.200500938
An operationally straightforward method for the amidation of aldehydes with economic ammonium chloride or amine hydrochloride salts enables the synthesis of various amides in good yield by using inexpensive copper sulfate or copper(I) oxide as a catalyst and aqueous tert-butyl hydroperoxide as an oxidant. Chiral amines can be used without detectable racemization.
S. C. Ghosh, J. S. Y. Ngiam, A. M. Seayad, D. T. Tuan, C. L. L. Chai, A. Chen, J. Org. Chem., 2012, 77, 8007-8015.
https://doi.org/10.1021/jo301252c
A mild and efficient oxidative amidation of aldehydes uses amine HCl salts and tert-butyl hydroperoxide as an oxidant in the presence of a copper catalyst.
W.-J. Yoo, C.-J. Li, J. Am. Chem. Soc., 2006, 128, 13064-13065.
https://doi.org/10.1021/ja064315b
A general and efficient method enables the synthesis of tertiary amides from readily available tertiary amines and anhydrides in the presence of FeCl2 as catalyst and tert-butyl hydroperoxide in water (T-Hydro) as oxidant. Mechanistic studies indicated that the in situ-generated α-amino peroxide of tertiary amine and iminium ion act as key intermediates.
Y. Li, L. Ma, F. Jia, Z. Li, J. Org. Chem., 2013, 78, 5638-5646.
https://doi.org/10.1021/jo400804p
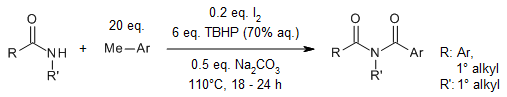
A direct coupling of NH-amides with methylarenes under iodine/aqueous TBHP conditions enables a metal-free synthesis of imides. The optimized conditions worked also very well with benzaldehydes and benzyl alcohol and furnished the corresponding imides in good to excellent yields.
H. Aruri, U. Singh, S. Kumar, M. Kushwaha, A. P. Gupta, R. A. Vishwakarma, P. P. Singh, Org. Lett., 2016, 18, 3638-3641.
https://doi.org/10.1021/acs.orglett.6b01684
A new α-amino acid esters formation via decarboxylation is distinguished by readily accessible starting materials, environmentally benign reaction conditions and waste streams, and wide substrate scope.
J. Zhang, J. Jiang, Y. Li, Y. Zhao, X. Wang, Org. Lett., 2013, 15, 3222-3225.
https://doi.org/10.1021/ol401139m
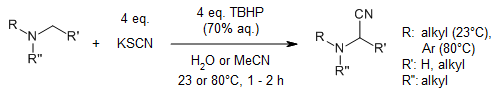
S-oxidation of potassium thiocyanate releases cyanide units that can be trapped in the presence of co-oxidized tertiary amines to form α-amino nitriles. These cyanations work in aqueous solutions without catalyst and without toxic byproducts.
A. Wagner, A. R. Ofial, J. Org. Chem., 2015, 80, 2848-2854.
https://doi.org/10.1021/jo502846c
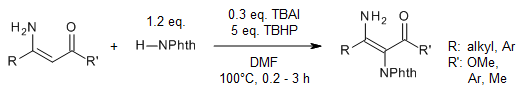
An efficient, safe, and environmentally friendly TBHP-mediated rearrangement of aryl/alkylidene malononitrile with anilines produces in situ HCN as the cyanide source for the synthesis of substituted α-aminonitriles and α-aminoamides in very good yields. This method features good functional group tolerance, and the in situ-generated HCN bypasses the use of an external cyanide source.
S. P. Bhoite, A. H. Bansode, G. Suryavanshi, J. Org. Chem., 2020, 85, 14858-14865.
https://doi.org/10.1021/acs.joc.0c01358
TBAI as catalyst and TBHP as oxidant enable a metal-free cross-coupling of enamines and electron-deficient amines through oxidative C(sp2)-N bond formation. This efficient organocatalytic synthesis of synthetically useful diaminoalkene derivatives features readily available starting materials and wide substrate scope.
Y. Yuan, W. Hou, D. Zhang-Negrerie, K. Zhaou, Y. Du, Org. Lett., 2014, 16, 5410-5113.
https://doi.org/10.1021/ol5026525
An iron-catalyzed acyl-azidation of alkenes provides unsymmetrical β-azido ketones under mild reaction conditions from aromatic aldehydes or aliphatic aldehydes as the acyl radical precursors, TMSN3 as the azido source, and TBHP as the initiator. The synthesized unsymmetrical β-azido ketones can be easily transformed into valuable functionalized compounds.
L. Ge, Y. Li, H. Bao, Org. Lett., 2019, 21, 256-260.
https://doi.org/10.1021/acs.orglett.8b03688
An oxidative copper-catalyzed arylation of various ring-size lactams with arylboronic acids gives N-arylated products in good yield without any additional bases, ligands, or additives.
T. Bathini, V. S. Rawat, B. Sreedhar, Synlett, 2015, 26, 1348-1351.
https://doi.org/10.1055/s-0034-1380741
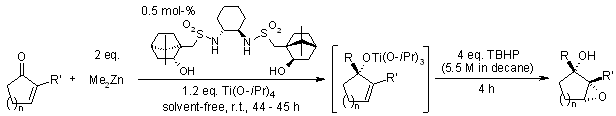
The catalytic asymmetric addition of alkyl groups to ketones under highly concentrated and solvent-free conditions permits reduction in catalyst loading by a factor of 2- to 40-fold compared with standard reaction conditions employing toluene and hexanes. Using cyclic conjugated enones, solvent-free asymmetric addition followed by a diastereoselective epoxidation using 5.5 M decane solution of tert-butyl hydroperoxide generated epoxy alcohols.
S.-J. Jeon, H. Li, P. J. Walsh, J. Am. Chem. Soc., 2005, 127, 16416-16425.
https://doi.org/10.1021/ja052200m
A catalytic asymmetric epoxidation reaction of various α,β-unsaturated esters via a conjugate addition of an oxidant using an yttirium-chiral biphenyldiol catalyst yielded the corresponding α,β-epoxy esters in up to 97% yield and 99% ee.
H. Kakei, R. Tsuji, T. Ohshima, M. Shibasaki, J. Am. Chem. Soc., 2005, 127, 8962-8963.
https://doi.org/10.1021/ja052466t
A gold(I)-catalyzed oxidative cleavage of alkenes with tert-butyl hydrogenperoxide (TBHP) as the oxidant in the presence of neocuproine afforded ketones or aldehydes as products.
D. Xing, B. Guan, G. Cai, Z. Fang, L. Yang, Z. Shi, Org. Lett., 2006, 8, 693-696.
https://doi.org/10.1021/ol052830t
With an easily accessible cinchona alkaloid catalyst, efficient enantioselective peroxidation and epoxidation have been successfully developed. Employing readily available α,β-unsaturated ketones and hydroperoxides, this novel reaction will open new possibilities in the asymmetric synthesis of chiral peroxides and epoxides.
X. Lu, Y. Liu, B. Sun, B. Cindric, L. Deng, J. Am. Chem. Soc., 2008, 130, 8134-8135.
https://doi.org/10.1021/ja802982h
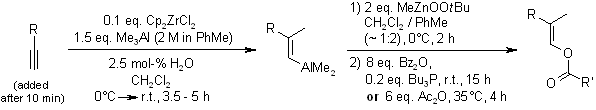
Stereodefined enol derivatives of aldehydes are prepared from terminal alkynes through Cp2ZrCl2-catalyzed methylalumination and subsequent oxygenation with peroxyzinc species and electrophilic trapping with carboxylic anydrides. The tandem carbometalation/oxygenation tolerates free and protected alcohols, heterocycles, olefins, and nitriles.
J. R. DeBergh, K. M. Spivey, J. M. Ready, J. Am. Chem. Soc., 2008, 130, 7828-7829.
https://doi.org/10.1021/ja803480b
A mild and efficient protocol for the synthesis of phenols from arylboronic acids in the presence of tert-butyl hydroperoxide is promoted by KOH. Products were obtained in good to excellent yields within several minutes.
S. Guo, L. Lu, H. Cai, Synlett, 2013, 24, 1712-1714.
https://doi.org/10.1055/s-0033-1339303
A copper iodide mediated cyanation of arylboronic acids and aryl iodides with ethyl (ethoxymethylene)cyanoacetate as cyanating agent involves a C(sp2)-CN bond cleavage and tolerates a wide range of functional groups to provide the corresponding aryl nitriles in good yields.
C. Qi, X. Hu, H. He, Synlett, 2016, 27, 1979-1982.
https://doi.org/10.1055/s-0035-1562112
Nitroarenes react with anions of tert-butyl and cumyl hydroperoxides in the presence of strong bases to form substituted o- and p-nitrophenols. The reaction usually proceeds in high yields and is of practical value as a method of synthesis and manufacturing of nitrophenols.
M. Makosza, K. Sienkiewicz, J. Org. Chem., 1998, 63, 4199-4208.
https://doi.org/10.1021/jo970726m
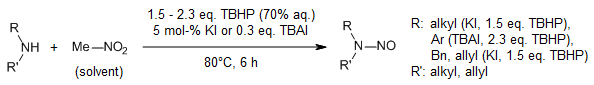
In a mild, iodide-catalyzed process to synthesize N-nitrosamines from amines and nitromethane using TBHP as the oxidant, the catalytic system succeeded in cleaving the carbon-nitrogen bond in nitromethane. This methodology uses commercially available, inexpensive catalysts and oxidants and has a wide substrate scope and operational simplicity.
J. Zhang, J. Jiang, Y. Li, X. Wan, J. Org. Chem., 2013, 78, 11366-11372.
https://doi.org/10.1021/jo401915t
A simple and effective copper-catalyzed oxidative cross-coupling of dimethylanilines with alkynes in the presence of tert-BuOOH allows the construction of propargylamines via a combination of sp3 C-H bond and sp C-H bond activations followed by C-C bond formation.
Z. Li, C.-J. Li, J. Am. Chem. Soc., 2004, 126, 11810-11811.
https://doi.org/10.1021/ja0460763
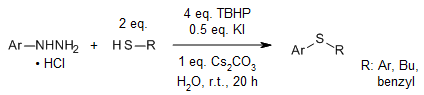
A mild, scalable iodine-mediated oxidative cross-coupling reaction of arylhydrazines and thiols enables the construction of thioethers (sulfides) in the absence of any transition metals or photocatalysts. A variety of unsymmetrical diaryl sulfides with broad substrate scope both on thiols and hydrazines were synthesized in high yields in water at room temperature.
F. Jafarpour, M. Asadpour, M. Azizzade, M. Ghasemi, S. Rajai-Daryasarei, Synthesis, 2020, 52, 727-734.
https://doi.org/10.1055/s-0039-1690757
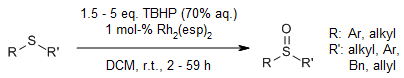
The dirhodium(II) carboxylate complex Rh2(esp)2 catalyzes the sulfoxidation of organic sulfides in the presence of tert-butyl hydroperoxide as the oxidant. As the rhodium catalyst is able to precipitate as a Rh2(esp)2-sulfoxide complex following the reaction, its separation and reuse is very convenient without considerable loss of activity.
L. Zhao, H. Zhang, Y. Wang, J. Org. Chem., 2016, 81, 129-134.
https://doi.org/10.1021/acs.joc.5b02400
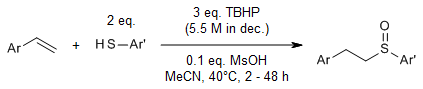
An oxidative variant of the thiol-ene reaction enables the direct addition of thiols to olefins to form sulfoxides in the presence of tert-butyl hydroperoxide as oxidant and methanesulfonic acid as catalyst. The latter is believed to catalyze the oxidation of the intermediate sulfide to the sulfoxide. Styrenes, acrylic acid derivatives, alkynes, and thiophenols gave the highest yields, while aliphatic olefins and thiols were less effective.
H.-L. Yue, M. Klussmann, Synlett, 2016, 27, 2505-2509.
https://doi.org/10.1055/s-0035-1562480
A metal-free room temperature decarboxylative cross-coupling between cinnamic acids and arylsulfonyl hydrazides provides (E)-vinyl sulfones. A regio- and stereoselective synthesis of 22 derivatives with diverse structural features has been achieved.
R. Singh, B. K. Allam, N. Singh, K. Kumari, S. K. Singh, K. N. Singh, Org. Lett., 2015, 17, 2656-2659.
https://doi.org/10.1021/acs.orglett.5b01037
A highly efficient, metal-free, and generally applicable iodine-catalyzed reaction of arylacetylenic acids and arylacetylenes with sodium sulfinates provides arylacetylenic sulfones.
J. Meesin, P. Katrun, C. Pareseecharoen, M. Pohmakotr, V. Reutrakul, D. Soorukram, C. Kuhakarn, J. Org. Chem., 2016, 81, 2744-2752.
https://doi.org/10.1021/acs.joc.5b02810
A metal-free direct condensation of sodium arylsulfinates and β,β-disubstituted nitroalkenes provides allylic sulfones in excellent yields with a broad substrate scope under mild conditions. The key step of this process was a Lewis base-promoted equilibrium between nitroalkenes and allylic nitro compounds, which contain more reactive C=C bonds toward sulfonyl radical addition.
X. Lei, L. Zheng, C. Zhang, X. Shi, Y. Chen, J. Org. Chem., 2018, 83, 1772-1778.
https://doi.org/10.1021/acs.joc.7b02595
A copper-catalyzed amidation of allylic and benzylic C-H is applicable to the coupling of a diverse set of hydrocarbon species with aryl, heteroaryl, and alkyl sulfonamides and is tolerant of a variety of functional groups.
G. Pelletier, D. A. Powell, Org. Lett., 2006, 8, 6031-6034.
https://doi.org/10.1021/ol062514u
NaI-catalyzed direct condensation of sulfonamides and formamides enables N-sulfonyl formamidine synthesis without hazardous reagents or transition-metal catalysts. The green methodology features high atom economy, operational simplicity, and good tolerance with diverse functional groups.
S. Chen, Y. Xu, X. Wan, Org. Lett., 2011, 13, 6152-6155.
https://doi.org/10.1021/ol2024604
A method for the synthesis of N-aroylated sulfoximines involves a manganese oxide promoted C-H activation of methyl arenes to form an aroyl intermediate which then reacts readily with N-chlorosulfoximines to form a series of valuable aroyl sulfoximine derivatives in high yields.
D. L. Priebbenow, C. Bolm, Org. Lett., 2014, 16, 1650-1652.
https://doi.org/10.1021/ol5003016
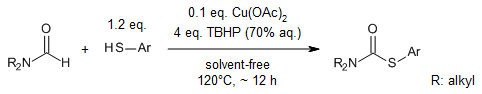
Cu(OAc)2-promoted TBHP oxidative coupling reaction of formamides with thiophenols gives S-aryl dialkyl thiocarbamates in high yield under solvent-free conditions through direct C-H bond activation of formamides.
Y.-q. Yuan, S.-r. Guo, J.-n. Xiang, Synlett, 2013, 24, 443-448.
https://doi.org/10.1055/s-0032-1318188
Tert-butyl hydroperoxide (TBHP) mediates a coupling of sulfonylhydrazides with thiols in the presence of CuBr2 as catalyst to afford thiosulfonates via a radical process.
G.-Y. Zhang, S.-S. Lv, A. Shoberu, J.-P. Zou, J. Org. Chem., 2017, 82, 9801-9807.
https://doi.org/10.1021/acs.joc.7b01121
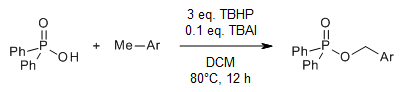
An efficient phosphorylation of C(sp3)-H bonds of readily available methyl arenes with diaryl phosphinic acids proceeds efficiently under transition-metal-free reaction conditions via Bu4NI-catalyzed dehydrogenative coupling to provide valuable organophosphorus compounds.
B. Xiong, G. Wang, C. Zhou, Y. Liu, P. Zhang, K. Tang, J. Org. Chem., 2018, 83, 993-999.
https://doi.org/10.1021/acs.joc.7b02422
A dual copper/photoredox-catalyzed approach for the construction of the P(O)-N bond from commercially available aromatic amines and P(O)-H compounds avoids toxic or corrosive reagents and does not require prefunctionalized substrates. The reaction has a broad substrate scope and is suitable for the synthesis of phosphonamides and phosphinamides.
K.-C. Yu, H. Li, Y.-H. Tu, H. Zhao, X.-G. Hu, Org. Lett., 2022, 24, 9130-9134.
https://doi.org/10.1021/acs.orglett.2c03860
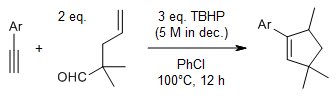
A metal-free oxidative decarbonylative [3+2] annulation of terminal alkynes with tertiary γ,δ-unsaturated aldehydes provides cyclopentenes, whereas the reaction of terminal alkynes with 2-methyl-2-arylpropanals provides indenes. The reactions offer broad substrate scope and excellent selectivity.
H.-X. Zou, Y. Li, X.-H. Yang, J. Xiang, J.-H. Li, J. Org. Chem., 2018, 83, 8581-8588.
https://doi.org/10.1021/acs.joc.8b01130
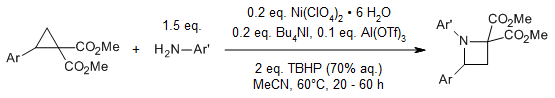
A relay catalysis strategy enables a [3+1]-annulation reaction between cyclopropane 1,1-diester and aromatic amines via lewis acid-catalyzed nucleophilic ring opening of cyclopropane 1,1-diester with aromatic amine and (hypo)iodite-catalyzed C-N bond formation. This reaction provides biologically important azetidines and tetrahydroquinolines.
J.-Q. Han, H.-H. Zhang, P.-F. Xu, Y.-C. Luo, Org. Lett., 2016, 18, 5212-5215.
https://doi.org/10.1021/acs.orglett.6b02430
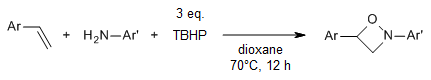
A [2+1+1] radical tandem cycloaddition of styrenes, arylamines, and tert-butyl hydroperoxide enables a regioselective synthesis of polysubstituted 1,2-oxazetidines. TBHP was employed in this conversion not only as the oxidant but also as the oxygen source.
W. Liu, C. Chen, P. Zhou, H. Tan, Org. Lett., 2017, 19, 5830-5832.
https://doi.org/10.1021/acs.orglett.7b02796
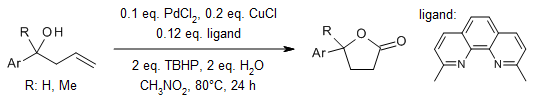
Palladium catalysis enables an unprecedented, highly effective synthesis of γ-lactones from homoallylic alcohols in one step. The protocol affords aryl, alkyl, and spiro γ-lactones directly from readily available homoallylic alcohols in good yields with excellent functional group tolerance and high chemoselectivity under mild conditions.
M. Zheng, P. Chen, L. Huang, W. Wu, H. Jiang, Org. Lett., 2017, 19, 5756-5759.
https://doi.org/10.1021/acs.orglett.7b02688
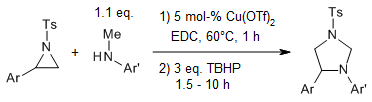
Copper efficiently catalyzes a reaction of N-sulfonylaziridines and N-alkylanilines in the presence of tert-butyl hydroperoxide to afford functionalized imidazolidines via nucleophilic ring opening, sp3 C-H functionalization, and C-N bond formation. The protocol can be used for the enantiospecific synthesis of imidazolidines with excellent optical purity.
M. Sengoden, A. Bhowmick, T. Punniyamurthy, Org. Lett., 2017, 19, 158-161.
https://doi.org/10.1021/acs.orglett.6b03458
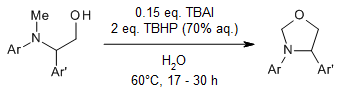
Tetrabutylammonium iodide as the catalyst and tert-butyl hydroperoxide in water (T-Hydro) as the oxidant enable a potential route for the construction of functionalized oxazolidines and imidazolidines via oxidative C(sp3)-H functionalization/C-O/C-N bonds formations. The reaction is simple, regioselective, and effective at moderate temperature with broad substrate scope.
V. Satheesh, M. Sengoden, T. Punniyamurthy, J. Org. Chem., 2016, 81, 9792-9801.
https://doi.org/10.1021/acs.joc.6b01850
A copper-catalyzed annulation of 1,3-dicarbonyl compound with diethylene glycol gives 2,3-disubstituted furans in the presence of tert-butyl peroxide (TBHP) via a sequential O- and C- functionalization of β-ketoester by diethylene glycol. Diethylene glycol serves as a environmentally friendly and cheap substitute of ethyne, that releases H2O and alcohol as clean wastes.
J.-T. Yu, B. Shi, H. Peng, S. Sun, H. Chu, Y. Jiang, J. Cheng, Org. Lett., 2015, 17, 3643-3645.
https://doi.org/10.1021/acs.orglett.5b01521
A copper-mediated intermolecular annulation of alkyl ketones and β-nitrostyrenes enables a regioselective synthesis of multisubstituted furan derivatives in good yields.
M. Ghosh, S. Mishra, A. Hajra, J. Org. Chem., 2015, 80, 5364-5368.
https://doi.org/10.1021/acs.joc.5b00704
An iodine catalyzed C (sp3)-H functionalization of tosylhydrazones with β-enamino esters under visible light irradiation provides tri- and tetra-substituted pyrroles.
N. N. K. Reddy, D. Rawat, S. Adimurthy, J. Org. Chem., 2018, 83, 9412-9421.
https://doi.org/10.1021/acs.joc.8b00878
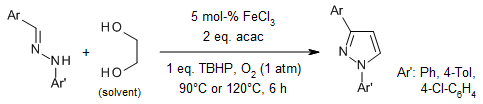
An iron-catalyzed route for the regioselective synthesis of 1,3- and 1,3,5-substituted pyrazoles from the reaction of diarylhydrazones and vicinal diols allows the conversions of a broad range of substrates.
N. Panda, A. K. Jena, J. Org. Chem., 2012, 77, 9401-9406.
https://doi.org/10.1021/jo301770k
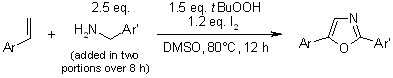
A facile one-pot, transition-metal-free process enables the synthesis of various polysubstituted oxazoles via t-BuOOH/I2-mediated domino oxidative cyclization from readily available starting materials under mild conditions.
H. Jiang, H. Huang, H. Cao, C. Qi, Org. Lett., 2010, 12, 5561-5563.
https://doi.org/10.1021/ol1023085
In a practical and simple synthesis of 2,5-disubstituted oxazoles via an iodine-catalyzed tandem oxidative cyclization, a wide range of common commercial aromatic aldehydes can be used as reaction substrates, which displayed excellent functional group compatibility.
C. Wan, L. Gao, Q. Wang, J. Zhang, Z. Wang, Org. Lett., 2010, 12, 3902-9305.
https://doi.org/10.1021/ol101596s
I2-catalyzed C-O bond formation and dehydrogenation with TBHP enables a general method for the synthesis of oxazolines and oxazoles from β-acylamino ketones. Depending on the base, either oxazolines or oxazoles were selectively produced.
W.-C. Gao, F. Hu, Y.-M. Huo, H.-H. Chang, X. Li, W.-L. Wei, Org. Lett., 2015, 17, 3914-3917.
https://doi.org/10.1021/acs.orglett.5b01933
A highly efficient copper-catalyzed tandem oxidative cyclization gives polysubstituted oxazoles from readily available starting materials under mild conditions. This is an attractive alternative method for the synthesis of oxazole derivatives.
C. Wang, J. Zhang, S. Wang, J. Fan, Z. Wang, Org. Lett., 2010, 12, 2338-2341.
https://doi.org/10.1021/ol100688c
An I2-catalyzed oxidative cross coupling of N-sulfonyl hydrazones with isocyanides in the presence of TBHP as terminal oxidant enables the synthesis of 5-aminopyrazoles through formal [4+1] annulation via in situ azoalkene formation. Notable features are a metal/alkyne-free strategy, atom economy, catalytic I2, broad functional group tolerance, good reaction yields and short time.
G. C. Senadi, W.-P. Hu, T.-Y. Lu, A. M. Garkhedkar, J. K. Vandavasi, J.-J. Wang, Org. Lett., 2015, 17, 1521-1524.
https://doi.org/10.1021/acs.orglett.5b00398
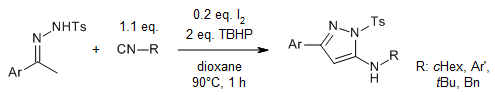
A general and metal-free synthesis of 1,3,5-trisubstituted 1,2,4-triazoles from hydrazones and aliphatic amines has been achieved under oxidative conditions via a cascade C-H functionalization, double C-N bonds formation, and oxidative aromatization sequence in the presence of iodine as catalyst.
Z. Chen, H. Li, W. Dong, M. Miao, H. Ren, Org. Lett., 2016, 18, 1334-1337.
https://doi.org/10.1021/acs.orglett.6b00277
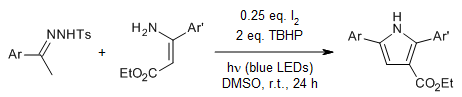
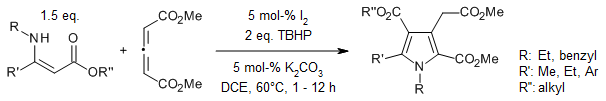
A iodine-catalyzed tandem Michael addition/oxidative annulation of allenes and enamines provides polysubstituted pyrroles in an efficient and highly regioselective way in good yields under mild conditions.
Y. Wang, C.-M. Jiang, H.-L. Li, F.-S. He, X. Luo, W.-P. Deng, J. Org. Chem., 2016, 81, 8653-8658.
https://doi.org/10.1021/acs.joc.6b01737
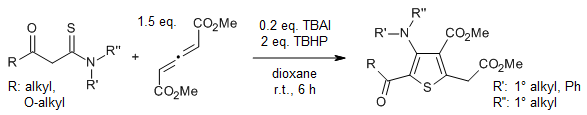
A TBAI/TBHP catalyst system enables a facile synthetis of highly functionalized 3-aminothiophenes from readily available allenes and thioamides in very good yields under mild reaction conditions via a tandem thio-Michael addition, oxidative annulation, and 1,2-sulfur migration pathway.
T. Han, Y. Wang, H.-L. Li, X. Luo, W.-P. Deng, J. Org. Chem., 2018, 83, 1538-1542.
https://doi.org/10.1021/acs.joc.7b02616
A Brønsted acid accelerated oxidative radical annulation of sulfonyl hydrazones with simple olefins provides six-membered heterocycles. The method offers a rapid and efficient approach to tetrahydropyridazines which are key structural motifs in pharmaceutically active compounds.
X. Zhong, J. Lv, S. Luo, Org. Lett., 2016, 18, 3150-3153.
https://doi.org/10.1021/acs.orglett.6b01360
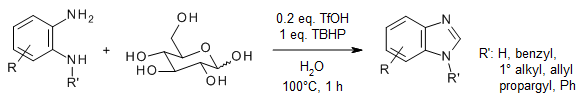
D-Glucose can be used as an efficient C1 synthon in the synthesis of benzimidazoles from o-phenylenediamines via an oxidative cyclization strategy. This method offers broad functional group tolerance, a biorenewable methine source, excellent reaction yields, a short reaction time, and water as an environmentally benign solvent.
D. Raja, A. Philips, P. Palani, W.-Y. Lin, S. Devikala, G. C. Senadi, J. Org. Chem., 2020, 85, 11531-11540.
https://doi.org/10.1021/acs.joc.0c01053
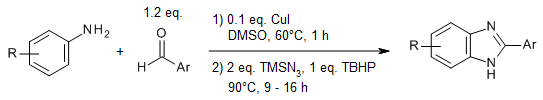
A one-pot, multicomponent reaction enables the transformation of commercial aryl amines, aldehydes, and azides into valuable benzimidazole structural units with wide substrate scope and diversity via an efficient copper-catalyzed amination of N-aryl imines, in which imine acts as a directing group by chelating to the metal center.
D. Mahesh, P. Sadhu, T. Punniyamurthy, J. Org. Chem., 2015, 80, 1644-1650.
https://doi.org/10.1021/jo502574u
A copper(II)-catalyzed oxidative cross-coupling of anilines, primary alkyl amines, and sodium azide provides benzimidazoles in the presence of TBHP at moderate temperature via a domino C-H functionalization, transimination, ortho-selective amination, and a cyclization sequence. The reaction offers broad substrate scope and functional group compatibility.
D. Mahesh, P. Sadhu, T. Punniyamurthy, J. Org. Chem., 2016, 81, 3227-3234.
https://doi.org/10.1021/acs.joc.6b00186
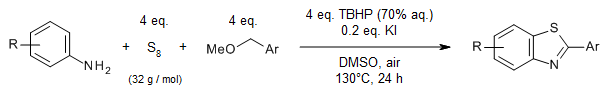
A selective, TBHP/KI-promoted C-O bond cleavage of ethers followed by annulation with anilines and elemental sulfur provides a wide range of 2-aryl-, 2-heteroaryl-, and 2-alkyl-substituted benzothiazoles with satisfactory yields and good functional group compatibility under transition-metal-free conditions.
J. Zhang, X. Zhao, P. Liu, P. Sun, J. Org. Chem., 2019, 84, 12596-12605.
https://doi.org/10.1021/acs.joc.9b02145
I2 and TBHP mediate a convenient synthesis of 2-acylbenzothiazoles in very good yields from acetophenones and benzothiazoles. The formal acylation of the benzothiazoles is achieved through a sequence involving formation of an aryl glyoxal, ring-opening of the benzothiazole followed by condensation of the amino group with the aryl glyoxal, cyclization and oxidation.
B. Wang, Q. Zhang, Z. Guo, K. Ablajan, Synthesis, 2020, 52, 3058-3064.
https://doi.org/10.1055/s-0040-1707204
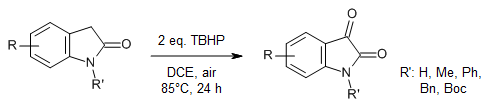
Recombination of alkyl radicals derived from indolin-2-ones with the tert-butylhydroperoxy radical affords 3-(tert-butylperoxy)indolin-2-one intermediates that can be further transformed into indoline-2,3-diones under air. This strategy provides a simple, effcient, and metal-free route to indolinediones.
W.-W. Ying, W.-M. Zhu, H. Liang, W.-T. Wei, Synlett, 2018, 29, 215-218.
https://doi.org/10.1055/s-0036-1589106
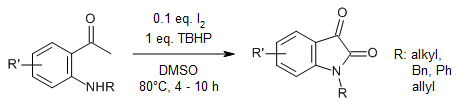
The use of a catalytic amount I2 and TBHP as stoichiometric oxidant enables a simple and atom economic one-pot synthesis of isatins from 2′-aminoacetophenones via oxidative amido cyclization of the sp3 C-H bond. In the presence of a stoichiometric amount of iodine, the reaction yields iodoisatines. The reaction proceeds through sequential iodination, Kornblum oxidation, and amidation in one pot.
A. Ilangovan, G. Satish, J. Org. Chem., 2014, 79, 4984-4991.
https://doi.org/10.1021/jo500550d
An efficient oxo-sulfonylation protocol enables the synthesis of N-sulfonylated indazolones from 2H-indazoles employing sulfinic acid as a sulfonylating agent in the presence of tert-butyl hydroperoxide (TBHP) under ambient air. A series of structurally diverse 1-sulfonylindazol-3(2H)-one derivatives were obtained in good yields.
P. Ghosh, S. Mondal, A. Hajra, Org. Lett., 2020, 22, 1086-1090.
https://doi.org/10.1021/acs.orglett.9b04617
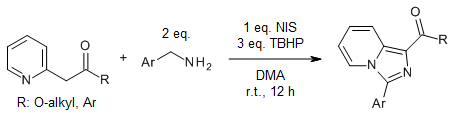
A metal-free sequential dual oxidative amination of C(sp3)-H bonds under ambient conditions affords imidazo[1,5-a]pyridines in very good yields. The reaction involves two oxidative C-N couplings and one oxidative dehydrogenation process with six hydrogen atoms removed.
Y. Yan, Y. Zhang, Z. Zha, Z. Wang, Org. Lett., 2013, 15, 2274-2277.
https://doi.org/10.1021/ol4008487
An efficient transition-metal-free oxidative cyclization reaction of alkynes with isatins enables a facile synthesis of structurally diverse 4-quinolones.
S.-F. Jiang, C. Xu, Z.-W. Zhou, Q. Zhang, X.-H. Wen, F.-C. Jia, A.-X. Wu, Org. Lett., 2018, 20, 4231-4234.
https://doi.org/10.1021/acs.orglett.8b01645
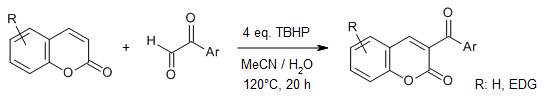
A simple switch in reaction conditions enables facile and efficient syntheses of various valuable 3-aryl- and 3-aroylcoumarins by direct arylation and aroylation of coumarins with glyoxals in a metal-free manner. This approach accommodates a broad substrate scope and high yields of the two types of cross-coupling reactions starting from identical starting materials.
A. Moazzam, M. Khodadadi, F. Jafarpour, M. Ghandi, J. Org. Chem., 2022, 87, 3630-3637.
https://doi.org/10.1021/acs.joc.1c02159
A facile approach allows the synthesis of 2-phenylquinazolines via a tandem reaction following sp3 C-H functionalization. Twenty-five examples of 2-phenylquinazolines were obtained from easily available 2-aminobenzophenones and benzylic amines with good to excellent yields.
J. Zhang, D. Zhu, C. Yu, C. Wan, Z. Wang, Org. Lett., 2010, 12, 2841-2843.
https://doi.org/10.1021/ol100954x
A facile and efficient method for the synthesis of 2-phenylquinazolines from 2-aminobenzophenones and benzylamines us catalyzed by ceric ammonium nitrate (CAN)-TBHP in acetonitrile. The corresponding 2-phenylquinazolines were obtained in good to excellent yields.
K. Karnakar, J. Shangkar, S. N. Murthy, K. Ramesch, Y. V. D. Nageshwar, Synlett, 2011, 1089-1096.
https://doi.org/10.1055/s-0030-1259960
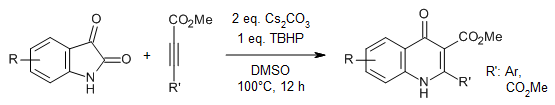
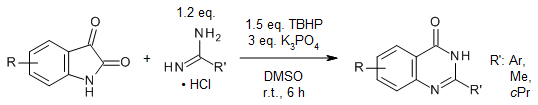
A synergetic tert-butyl hydroperoxide/K3PO4-promoted oxidative cyclization enables a facile synthesis of various functionalized quinazolin-4(3H)-ones from commercially available isatins and amidine hydrochlorides at room temperature.
F.-C. Jia, Z.-W. Zhou, C. Xu, Y.-D. Wu, A.-X. Wu, Org. Lett., 2016, 18, 2942-2945.
https://doi.org/10.1021/acs.orglett.6b01291
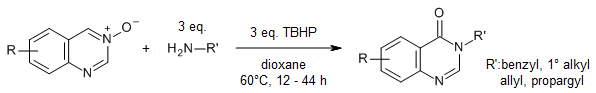
An efficient and facile reaction of quinazoline-3-oxides with primary amines provides a broad range of quinazolin-4(3H)-ones under metal-free and mild reaction conditions employing tert-butyl hydroperoxide as the oxidant. Remarkably, a precursor for the synthesis of bioactive evodiamine and rutaempine was conveniently obtained in good yield.
J. Luo, J. Wan, L. Wu, L. Yang, T. Wang, J. Org. Chem., 2022, 87, 9864-9874.
https://doi.org/10.1021/acs.joc.2c00898
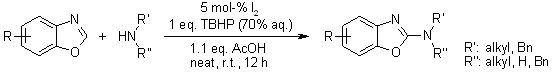
A facile metal-free oxidative amination of benzoxazole by activation of C-H bonds with secondary or primary amines in the presence of catalytic iodine in aqueous tert-butyl hydroperoxide proceeds smoothly at ambient temperature under neat reaction condition to furnish products in high yields. This user-friendly method produces only tertiary butanol and water as byproducts.
M. Lamani, K. R. Prabhu, J. Org. Chem., 2011, 76, 7938-7944.
https://doi.org/10.1021/jo201402a
Catalytic amounts of tetrabutylammoniumiodide (TBAI), aqueous solutions of H2O2 or TBHP as co-oxidant enabled an efficient transition-metal-free amination of benzoxazoles under mild reaction conditions, to yield highly desirable 2-aminobenzoxazoles in good yields. First mechanistic experiments indicate the in situ iodination of the secondary amine as the putative mode of activation.
T. Froehr, C. P. Sindlinger, U. Kloeckner, P. Finkbeiner, B. J. Nachtsheim, Org. Lett., 2011, 13, 3754-3757.
https://doi.org/10.1021/ol201439t
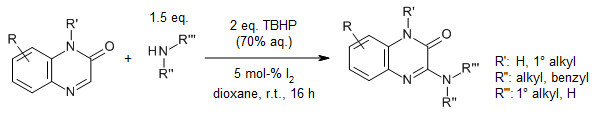
A metal-free cross-dehydrogenative coupling between quinoxalinones and amines in the presence of catalytic iodine and aqueous tert-butyl hydroperoxide as the terminal oxidant provides 3-aminoquinoxalinones in good yields in dioxane as solvent. The reaction is highly versatile and exhibits good functional group tolerance with a range of primary and secondary amines.
A. Gupta, M. S. Deshmuk, N. Jain, J. Org. Chem., 2017, 82, 4748-4792.
https://doi.org/10.1021/acs.joc.7b00464
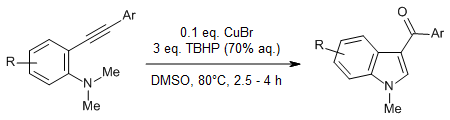
Cu-catalyzed sp3 C-H bond activation α to the nitrogen atom of o-alkynylated N,N-dimethylamines followed by an intramolecular nucleophilic attack with the alkyne, using an aqueous solution of tert-butyl hydroperoxide (TBHP) as the oxidant, enables a tandem catalytic synthesis of 3-aroylindoles. In this synthesis, both C-C and C-O bonds are installed at the expense of two sp3 C-H bond cleavages.
A. Gogoi, S. Guin, S. K. Rout, B. K. Patel, Org. Lett., 2013, 15, 1802-1805.
https://doi.org/10.1021/ol400692b
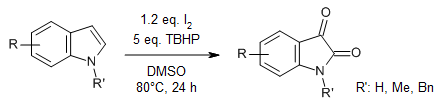
An I2/TBHP-mediated oxidation of commercially available indoles affords isatins in moderate to good yields.
Y. Zi, Z.-J. Cai, S.-Y. Wang, S.-J. Ji, Org. Lett., 2014, 16, 3094-3097.
https://doi.org/10.1021/ol501203q
A copper/acid-catalyzed oxidative carbamoylation of various electron-deficient nitrogen heteroarenes (isoquinolines, quinolines, pyridines, phenanthridines, quinoxalines, and phthalazines) with hydrazinecarboxamide hydrochlorides provides structurally diverse nitrogen-heteroaryl carboxamides as single regioisomers in moderate to excellent yields.
Z.-Y. He, C.-F. Huang, S.-K. Tian, Org. Lett., 2017, 19, 4850-4853.
https://doi.org/10.1021/acs.orglett.7b02312
An iron-catalyzed C(sp3)-H oxidation, intramolecular C-N bond formation, and aromatization enables an efficient synthesis of quinazolines from 2-alkylamino N-H ketimine derivatives, which can be prepared by addition of various organometallic reagents to 2-alkylamino benzonitriles.
C.-y. Chen, F. He, G. Tang, H. Yuan, N. Li, J. Wang, R. Faessler, J. Org. Chem., 2018, 83, 2395-2401.
https://doi.org/10.1021/acs.joc.7b02943
A Cu-catalyzed denitrogenative transannulation of 3-aminoindazoles provides diverse functionalized 3-aminobenzothiophenes and 1-aminoisoquinolines. This transformation proceeds via an "extrude-and-sew" strategy with an unprecedented radical reactivity of 3-aminoindazoles.
Y. Zhou, Y. Wang, Y. Lou, Q. Song, Org. Lett., 2019, 21, 8869-8873.
https://doi.org/10.1021/acs.orglett.9b02288
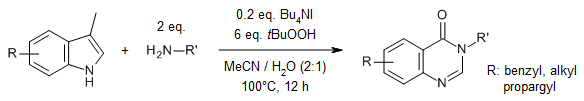
An n-Bu4NI-catalyzed reaction of 3-methylindoles with primary amines using TBHP as the unique oxidant provides broad range of quinazolinones in very good yields. The reaction involves oxygenation, nitrogenation, ring-opening, and recyclization.
J. He, J. Dong, L. Su, S. Wu, L. Liu, S.-F. Yin, Y. Zhou, Org. Lett., 2020, 22, 2522-2526.
https://doi.org/10.1021/acs.orglett.0c00271
An annulation of 2-cyanoaryl acrylamides via C=C double bond cleavage enables a facile and efficient synthesis of functionalized 4-amino-2-quinolones, which are important N-heterocycles. In this transformation, a THF radical plays a crucial role.
W.-J. Xia, T.-G. Fan, Z.-W. Zhao, X. Chen, X.-X. Wang, Y.-M. Li, Org. Lett., 2021, 23, 6158-6163.
https://doi.org/10.1021/acs.orglett.1c02281
Reactions between readily available o-hydroxyphenyl enaminones and various alkenes enable a a step economical and general synthesis of 3-vinyl chromones.
L. Fu, Z. Xu, J.-P. Wan, Y. Liu, Org. Lett., 2020, 22, 9518-9523.
https://doi.org/10.1021/acs.orglett.0c03548
The use of the Langlois reagent enables an efficient and regioselective synthesis of various 3-(trifluoromethyl)chromones from enamino ketones. This convenient procedure offers high product purity and yield.
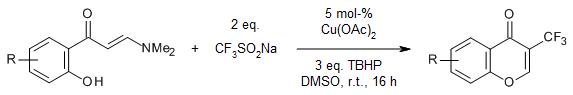
P. Thota, K. Sheelam, S. Kottawar, K. Shivakumar, M. Kaliyaperumal, S. Yennam, M. Behera, Synlett, 2022, 33, 1660-1664.
https://doi.org/10.1055/a-1906-3382
Tert-Butyl hydroperoxide promotes an oxidative annulation reaction of isatins with 2-(trimethylsilyl)aryl triflates for the convenient synthesis of acridone derivatives. The reaction may proceed via consecutive Baeyer-Villiger-type rearrangement followed by an intermolecular cyclization. This convenient method offers broad substrate scope and good functional group tolerance.
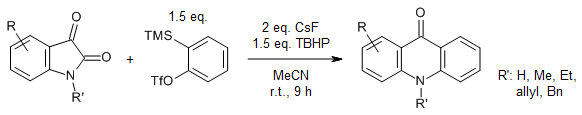
M. Luo, N. Dong, M. Zhu, Y. Wang, C. Xu, G. Yin, J. Org. Chem., 2023, 88, 9419-9423.
https://doi.org/10.1021/acs.joc.3c00250
Aqueous tert-butyl hydroperoxide (70%) is an inexpensive reagent for the regioselective and chemoselective deprotection of terminal acetonide groups. Various acetonide derivatives furnish the corresponding deprotected diols in good yields, while a large number of acid labile protecting functional groups and other functional moieties were found to be unaffected under the conditions.
M. R. Maddani, K. R. Prabhu, Synlett, 2011, 821-825.
https://doi.org/10.1055/s-0030-1259917
A mild and practical protocol for the copper-mediated trifluoromethylation of aryl and heteroaryl boronic acids using NaSO2CF3 (Langlois’ reagent) and TBHP proceeds at room temperature under ambient conditions. The products can be readily purified by extraction or column chromatography.
Y. Ye, S. A. Künzi, M. S. Sanford, Org. Lett., 2012, 14, 4979-7981.
https://doi.org/10.1021/ol3022726
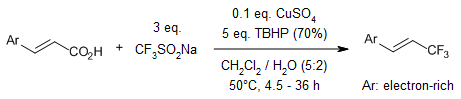
A copper-catalyzed decarboxylative trifluoromethylation of various α,β-unsaturated carboxylic acids was achieved by using a stable and inexpensive solid, sodium trifluoromethanesulfinate (CF3SO2Na, Langlois reagent). In addition, an iron-catalysis enables a difluoromethylation of aryl-substituted acrylic acids by using zinc difluoromethanesulfinate (DFMS, (CF2HSO2)2Zn, Baran reagent) via a similar radical process.
Z. Li, Z. Cui, Z.-Q. Liu, Org. Lett., 2013, 15, 406-409.
https://doi.org/10.1021/ol3034059
Halosulfonylation of terminal alkynes was achieved with sulfonylhydrazides as the sulfonyl precursor and inexpensive iron halide as halide source in the presence of TBHP to yield (E)-β-chloro and bromo vinylsulfones regio- and stereoselectively.
X. Li, X. Shi, M. Fang, X. Xu, J. Org. Chem., 2013, 78, 9499-9504.
https://doi.org/10.1021/jo401581n
A TBHP/TBAI-mediated reaction of propargyl alcohols with sulfonyl hydrazides in the presence of HOAc provides allenyl sulfones in good yields in a short reaction time via HOAc-promoted sulfonohydrazide intermediate formation, sequential C-O, C-N, and N-S bond cleavage, and C-S bond formation. This reaction shows highly functional group compatibility and excellent regioselectivity.
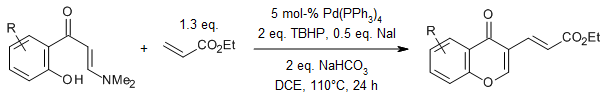
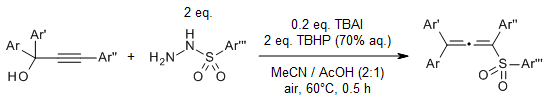
Z. Yang, W.-J. Hao, S.-L. Wang, J.-P. Zhang, B. Jiang, G. Li, S.-J. Tu, J. Org. Chem., 2015, 80, 922
https://doi.org/10.1021/acs.joc.5b01684
Quoted
from:https://www.organic-chemistry.org/chemicals/oxidations/tert-butylhydroperoxide.shtm
Aladdin:https://www.aladdinsci.com