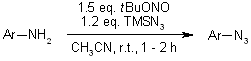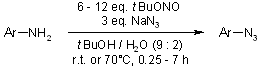Tert-Butyl Nitrite (TBN)
Product Manager:Nick Wilde
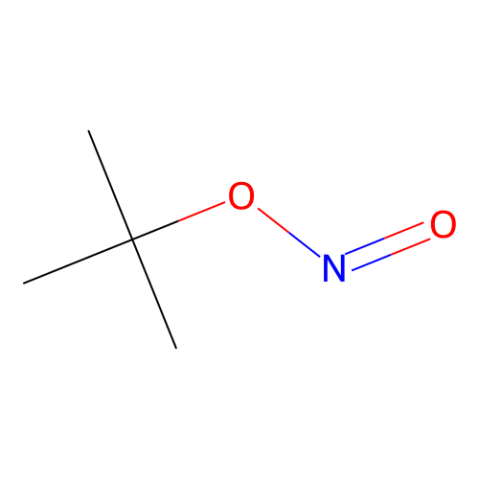
Recent Literature
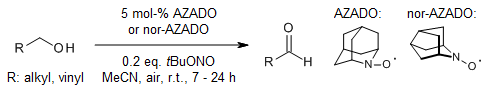
The use of tert-butyl nitrite as the co-catalyst in a 2-azaadamantane-N-oxyl(AZADO)- and 9-azanoradamantane-N-oxyl(nor-AZADO)-catalyzed efficient aerobic oxidation of primary alcohols in MeCN instead of the previously reported AcOH provides the corresponding aldehydes selectively. The addition of saturated aqueous NaHCO3 after the completion of the reaction suppresses the overoxidation of the product during the workup.
M. Shibuya, K. Furukawa, Y. Yamamoto, Synlett, 2017, 28, 1554-1557.
https://doi.org/10.1055/s-0036-1588155
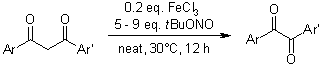
A selective C-C bond cleavage of 1,3-diketones affords 1,2-diketones in high yields under mild reaction conditions in air by the use of FeCl3 as the catalyst and tert-butyl nitrite(TBN) as the oxidant without the use of solvent. The possible reaction mechanism is discussed. This protocol provides an expeditious route to useful 1,2-diketones.
L. Huang, K. Cheng, B. Yao, Y. Xie, Y. Zhang, J. Org. Chem., 2011, 76, 5732-5737.
https://doi.org/10.1021/jo200840y
The synthesis of aromatic azides from the corresponding amines is accomplished under mild conditions with tert-butyl nitrite and azidotrimethylsilane. 1,4-Disubstituted 1,2,3-triazoles were obtained in excellent yields from various aromatic amines without the need for isolation of the azide intermediates.
K. Barral, A. D. Moorhouse, J. E. Moses, Org. Lett., 2007, 9, 1809-1811.
https://doi.org/10.1021/ol070527h
Aliphatic and aromatic aldehydes gave acyl azides by reaction with iodine azide at 0 - 25°C. If the reaction is performed at reflux Curtius rearrangement occurs and carbamoyl azides are obtained directly from the aldehyde in 70-97% yield.
L. Marinescu, J. Thinggaard, I. B. Thomsen, M. Bols, J. Org. Chem., 2003, 68, 9453-9455.
https://doi.org/10.1055/s-2005-869974
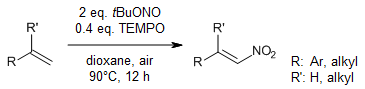
A wide range of olefins with diverse functionalities has been nitrated in synthetically useful yields in a single step under metal-free conditions. This transformation is operationally simple and exhibits excellent E-selectivity. Furthermore, site selective nitration in a complex setup makes this method advantageous.
S. Maity, T. Naveen, U. Sharma, D. Maiti, Org. Lett., 2013, 15, 3384-3387.
https://doi.org/10.1021/ol401426p
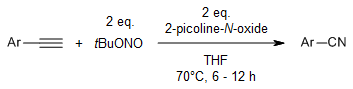
A metal-free C≡C bond cleavage of terminal alkynes in the presence of tBuONO as a powerful nitrogenating agent provides a vast range of nitriles containing aryl, heteroaryl, and natural product derivatives.
U. Dutta, D. W. Lupton, D. Maiti, Org. Lett., 2016, 18, 860-863.
https://doi.org/10.1021/acs.orglett.6b00147
In situ nitrosation of anilines followed by reduction with ascorbic acid to form aryl radicals and thiolation with disulfides provided aryl sulfides. This mild, metal-free synthesis of aryl sulfides proceeded smoothly without heating or irradiation. This strategy can be expanded to the synthesis of aryl selenides.
M.-j. Bu, G.-p. Lu, C. Cai, Synlett, 2015, 26, 1841-1846.
https://doi.org/10.1055/s-0034-1378738
A transition metal/ligand-free disulfuration of anilines can be performed under mild conditions and exhibits broad scope across the aniline substrate and disulfur transfer reagent classes (dithiosulfonate or tetrasulfide). This reaction can be considered as a reductive disulfuration variation of the classic Sandmeyer reaction.
S. Chen, S. Cao, C. Liu, B. Wang, X. Ren, H. Huang, Z. Peng, X. Wang, Org. Lett., 2021, 23, 7428-7433.
https://doi.org/10.1021/acs.orglett.1c02636
A catalytic amount of inexpensive salicylic acid promotes a straightforward and scalable synthesis of diphenyl arylphosphonates from anilines and triphenyl phosphite at 20°C within 1-2 h. The reaction proceeds via radical-radical coupling and tolerates a wide range of functional groups.
M. Estruch-Blasco, D. Felipe-Blanco, I. Bosque, J. C. González-Gómez, J. Org. Chem., 2020, 85, 14473-14485.
https://doi.org/10.1021/acs.joc.0c00795
Tert-Butyl nitrite (TBN) is a multitask reagent for the controlled synthesis of N-nitrosoamides from N-alkyl amides, hydrolysis of N-methoxyamides to carboxylic acids, metal-free benzocoumarin synthesis from ortho-aryl-N-methoxyamides, and Ru-catalyzed isocoumarin synthesis.
S. L. Yedage, B. M. Bhanage, J. Org. Chem., 2017, 82, 5769-5781.
https://doi.org/10.1021/acs.joc.7b00570
A facile oxidative heterocyclization of commercially available amines and tert-butyl nitrite with alkynes or alkenes providies isoxazoles or isoxazolines. This reaction is highly efficient, regiospecific, operationally simple, mild, and tolerates a variety of functional groups.
X.-W. Zhang, X.-L. He, N. Yan, H.-X. Zhang, X.-G. Hu, J. Org. Chem., 2020, 85, 15726-15735.
https://doi.org/10.1021/acs.joc.0c02281
A facile oxidative heterocyclization of commercially available amines and tert-butyl nitrite with alkynes or alkenes providies isoxazoles or isoxazolines. This reaction is highly efficient, regiospecific, operationally simple, mild, and tolerates a variety of functional groups.
X.-W. Zhang, X.-L. He, N. Yan, H.-X. Zhang, X.-G. Hu, J. Org. Chem., 2020, 85, 15726-15735.
https://doi.org/10.1021/acs.joc.0c02281
An unprecedented cross-coupling reaction between copper carbenes and nitroso radicals provides various isoxazolines via construction of C-C, C-O, and C=N bonds in a one-pot process. The convenient method offers mild reaction conditions and wide substrate scope.
R. Chen, Y. Zhao, S. Fang, W. Long, H. Sun, X. Wan, Org. Lett., 2017, 19, 5896-5899.
https://doi.org/10.1021/acs.orglett.7b02885
Visible-light mediates a photocatalytic regioselective [2+2+1] radical annulation reaction of alkenes, tert-butyl nitrite, and gem-dihalides to provide isoxazolines in good yields under mild conditions. Whereas gem-dihalides serve as C1 synthons, cheap tert-butyl nitrite acts as an ideal "N-O" synthon.
J. Liu, S. Tang, H. Xu, R. Zhang, J. Zhao, P. Zhang, P. Li, Org. Lett., 2022, 24, 9366-9369.
https://doi.org/10.1021/acs.orglett.2c03635
The combination of gold catalysis and radical chemistry enables the synthesis of 5-oxazole ketones from internal N-propargylamides in the presence of 4-MeO-TEMPO as an oxidant. The desired 5-oxazole ketones were provided in good yields with an excellent functional group compatibility under mild conditions.
H. An, S. Mai, Q. Xuan, Y. Zhou, Q. Song, J. Org. Chem., 2019, 84, 401-408.
https://doi.org/10.1021/acs.joc.8b02334
A one-pot protocol for the construction of fluoroalkylated isoxazoles directly from commercially available amines and alkynes is regioselective, scalable, operationally simple, mild, and tolerant of a broad range of functional groups. Preliminary mechanistic investigations reveal that the transformation involves an unprecedented Cu-catalyzed cascade sequence involving RfCHN2.
X.-W. Zhang, W.-L. Hu, S. Chen, X.-G. Hu, Org. Lett., 2018, 20, 860-863.
https://doi.org/10.1021/acs.orglett.7b04028
A metal-free, cascade regio- and stereoselective trifluormethyloximation, cyclization, and elimination strategy of readily available α,β-unsaturated carbonyl compounds with CF3SO2Na and tBuONO provides a wide variety of 4-(trifluoromethyl)isoxazoles. Mechanistic studies revealed a radical pathway for the reaction.
P. Pattanayak, T. Chatterjee, J. Org. Chem., 2023, 88, 5420-5430.
https://doi.org/10.1021/acs.joc.2c03053
KI mediates a noble-metal-free oxidative cyclization of enamines with tBuONO as an aminating reagent and oxidant. This formal [4+1] cycloamination reaction provides imidazole-4-carboxylic derivatives with wide substrate scope and good functional tolerance.
Y. Li, J. Qiu, P. Gao, L. Zhai, Z.-J. Bai, H.-J. Chen, J. Org. Chem., 2022, 87, 15380-15388.
https://doi.org/10.1021/acs.joc.2c01943
Tert-Butyl nitrite promotes an oxidative metal-free intermolecular sulfonamination of alkynylamines with sulfinic acids to provide substituted sulfonyl pyrroles via tandem addition/cyclization. Various substituted sulfonyl pyrroles are formed in good yields.
Z. Qi, Y. Jiang, Y. Wang, R. Yan, J. Org. Chem., 2018, 83, 8607-8614.
https://doi.org/10.1021/acs.joc.8b00741
A convenient oxidation of oxindoles with molecular oxygen in the presence of tert-butyl nitrite as an additive enables a mild and metal-free synthesis of isatins without the need for any catalyst or base.
W.-T. Wei, W.-W. Ying, W.-M. Zhu, Y. Wu, Y.-L. Huang, Y.-Q. Cao, Y.-N. Wang, H. Liang, Synlett, 2017, 28, 2307-2310.
https://doi.org/10.1055/s-0036-1590965
A tandem nitrosation/cyclization reaction of N-aryl cyanoacetamides with tert-butyl nitrite provides quinoxalin-2-ones in good yields with good functional group tolerance. The dehydrogenative N-incorporation is achieved through a sequence of nitrosation, tautomerization, and cyclization.
F. Wang, B.-L. Hu, L. Liu, H.-Y. Tu, X.-G. Zhang, J. Org. Chem., 2017, 82, 11247-11252.
https://doi.org/10.1021/acs.joc.7b01930
Quoted
from:https://www.organic-chemistry.org/chemicals/oxidations/tert-butyl-nitrite.shtm
Aladdin:https://www.aladdinsci.com