Tetrabromomethane, CBr4
Product Manager:Nick Wilde
Name Reactions
![]()
Appel Reaction
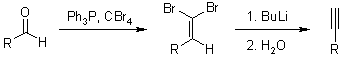
Corey-Fuchs Reaction
Recent Literature

A facile synthesis of aryl carboxylic acids from aryl ketones by aerobic photooxidation using the inexpensive and easily handled CBr4 as catalyst is applicable to inert compounds under usual photo-irradiation conditions, and appears very attractive for the expansion of the Norrish Type I reaction.
S.-i. Hirashima, T. Nobuta, N. Tada, A. Itoh, Synlett, 2009, 2017-2019.
https://doi.org/10.1055/s-0029-1217524
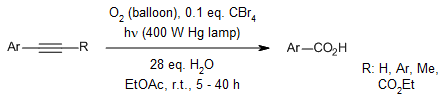
The presence of catalytic amounts of carbon tetrabromide enables an aerobic photooxidative cleavage of carbon-carbon triple bonds to carboxylic acids under photoirradiation.
T. Yamaguchi, T. Nobuta, Y. Kudo, S.-i. Hirashima, N. Tada, T. Miura, A. Itoh, Synlett, 2013, 24, 607-610.
https://doi.org/10.1055/s-0032-1318308
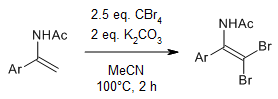
A base-promoted dibromination of enamides using CBr4 as a bromine source provides β,β-dibrominated secondary enamides. These novel products can be readily transformed to 5-Br oxazoles via Cu(I) catalyzed intramolecular cyclization in good yields.
J. Ma, Q. Zou, C. Wang, G. Yin, F. Li, J. Org. Chem., 2022, 87, 15670-15678.
https://doi.org/10.1021/acs.joc.2c01927
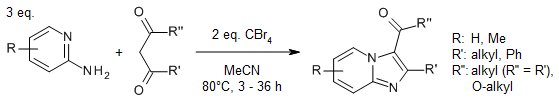
A carbon tetrabromide mediated oxidative carbon-nitrogen bond formation of 2-aminopyridines or 2-aminopyrimidines with β-keto esters or 1,3-diones provides substituted imidazo[1,2-α]pyridines or imidazo[1,2-α]pyrimidines under mild and metal-free conditions.
C. Huo, J. Tang, H. Xie, Y. Wang, J. Dong, Org. Lett., 2016, 18, 1016-1019.
https://doi.org/10.1021/acs.orglett.6b00137
Quoted
from:https://www.organic-chemistry.org/chemicals/oxidations/carbon-tetrabromide.shtm
Aladdin:https://www.aladdinsci.com