Tetrahydroxydiboron/Bis-Boric Acid/BBA/B2(OH)4
Sandra Forbes
Product Manager


Tetrahydroxydiboron is a reducing agent, that for example slowly produces hydrogen gas in water.
Recent Literature

A rhodium-catalyzed transfer hydrogenation of functionalized arenes in the presence of tetrahydroxydiboron offers good functional group tolerance, operational simplicity and controllable chemoselectivity. The general applicability of this procedure is demonstrated by the selective hydrogenation of a range of arenes, including functionalized benzenes, biphenyls, and polyaromatics.
Y. Wang, Z. Chang, Y. Hu, X. Lin, X. Dou, Org. Lett., 2021, 23, 1910-1914.

A metal-free reduction of nitro aromatics is mediated by tetrahydroxydiboron under mild conditions in water as solvent to provide various aromatic amines with good functional group tolerance and in good yields.
D. Chen, Y. Zhou, H. Zhou, S. Liu, Q. Liu, K. Zhang, Y. Uozumi, Synthesis, 2018, 50, 1765-1768.
The use of tetrahydroxydiboron as reductant and 4,4′-bipyridine as organocatalyst enables a metal-free and highly chemoselective reduction of aromatic nitro compounds within 5 min at room temperature. Under optimal conditions, sensitive functional groups, such as vinyl, ethynyl, carbonyl, and halogen were tolerated.
M. Jang, T. Lim, B. Y. Park, M. S. Han, J. Org. Chem., 2022, 87, 910-919.
B2(OH)4 mediates a reductive transamidation reaction between N-acyl benzotriazoles and organic nitro compounds or NaNO2 under mild conditions in H2O as solvent. N-Deuterated amides can be synthesized when conducting the reaction in D2O. A reasonable reaction mechanism involves bond metathesis between the AcBt amide and an amino boric acid intermediate.
J. Bai, S. Li, R. Zhu, Y. Li, W. Li, J. Org. Chem., 2023, 88, 3714-3723.
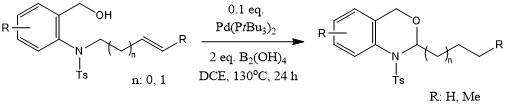
An effient tandem process consisting of palladium-catalyzed double-bond isomerization of long-chain olefins followed by intramolecular cyclization promoted by B2(OH)2 provides benzo-fused oxazaheterocycles. This strategy also provides rapid access to pyrido[3,4-b]indoles, trans-2-olefins, and enamides with high regio- and stereoselectivity.
L. Ding, Y.-N. Niu, X.-F. Xia, J. Org. Chem., 2021, 86, 10032-10042.
A copper-catalyzed one-pot reaction of 2-nitrobenzonitriles and various carbonyl compounds in the presence of diboronic acid as a reductant provides 2,3-dihydroquinazolin-4(1H)-ones with good functional-group tolerance in good yields under mild conditions.
Q. Liu, Y. Sui, Y. Zhang, K. Zhang, Y. Chen, H. Zhou, Synlett, 2020, 31, 275-279.
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/tetrahydroxydiboron-bba.shtm
Aladdinsci: https://www.aladdinsci.com/