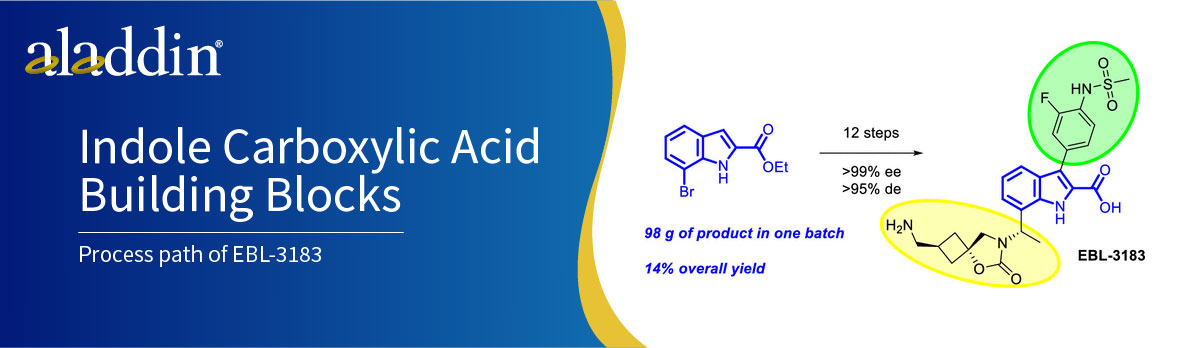
The process path of EBL-3183: from indole to preclinical inhibitor
Product Manager:Nick Wilde
Background
Metallo-β-lactamases (MBLs) are regarded as major determinants of carbapenem antibiotic resistance. Therefore, the search for new MBL inhibitors has become an urgent task at present. Recently, Prof. Andrei Baran and his team published an article in Org. Process Res. Dev, detailing the synthetic pathway of MBL inhibitor "EBL-3183" and how to optimize the synthesis process to improve the yield and purity to meet the needs of preclinical studies.
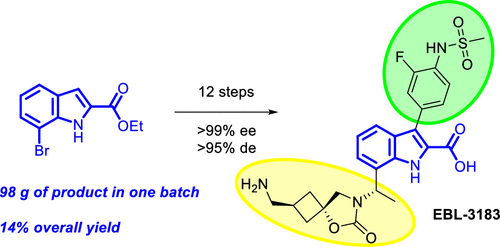
About EBL-3183
EBL-3183, a novel inhibitor of metallo-β-lactamases (MBLs), effectively interferes with MBL activity and enhances the therapeutic effect of antibiotics. It is currently in the preclinical research stage.
EBL-3183 is a novel compound containing an indole carboxylic acid (InC) scaffold with excellent activity. Although a previous candidate compound, InC 1, demonstrated superior inhibitory activity in vitro and in vivo, it performed poorly in terms of safety. Therefore, the team turned to the analog InC 2, which showed a better safety profile in vivo compared to InC 1 and also exhibited good potentiation of carbapenems.
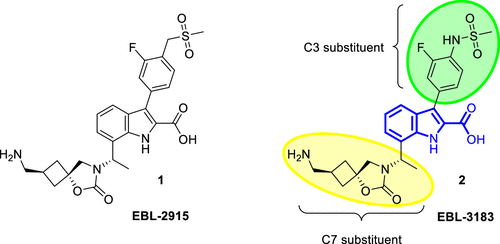
Figure 1. Structure of indole carboxylate metallo-β-lactamase inhibitors InC 1 (EBL-2915) and InC 2 (EBL-3183).
Synthesis Strategy
The research team has successfully synthesized the candidate compound EBL-3183 via a novel synthetic route and has achieved production on a kilogram scale. This synthetic process involves the introduction of chirality via an Elman-assisted approach and ruthenium-catalyzed stereoselective reduction using indole-2-carboxylates as starting materials. The key spirocyclic cyclobutane moiety is then derived from the delicate assembly of epoxide structural units, which can be obtained in diastereomerically pure form, thus facilitating the synthesis process.
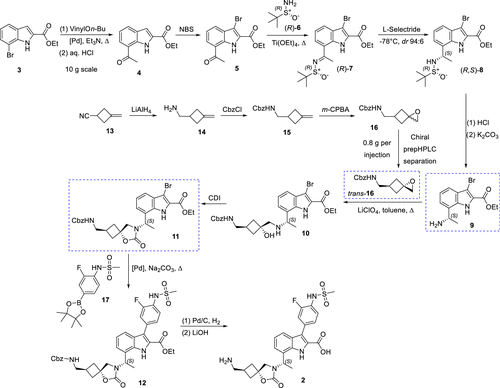
Figure 2. Medicinal Chemistry Route to InC 2 (EBL-3183)
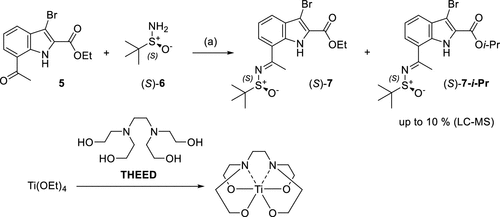
The preparation of (S)-7 is a crucial step in the synthesis process, which requires extremely selective reducing conditions. With better selective reducing agents and catalytic conditions, the separation problem of isomers can be effectively avoided, while the crystallinity of the product is enhanced, which in turn simplifies the subsequent purification operation. In addition, in order to ensure the successful synthesis of (S)-7, the quality of titanium reagent must be strictly controlled to prevent the generation of impurities.
Figure 3. Ti(OEt)4-Promoted Synthesis of the Sulfinyl Imine (S)-7a
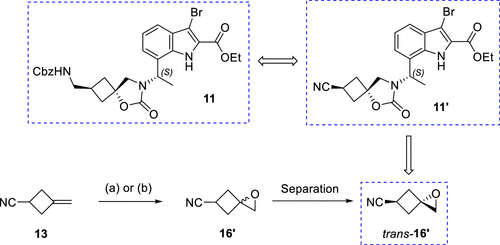
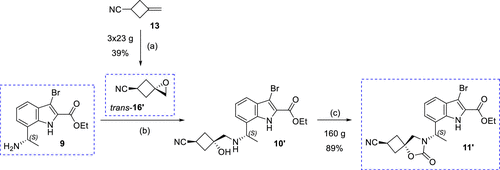
One of the keys to achieving large-scale synthesis of the target compound, InC 2, is the ability to scalably achieve stereoselectivity for the preparation of the key spiro-ring-conjugated cyclobutane-epoxide trans-16. trans-16', a cyclobutane building block, was successfully prepared by epoxidizing the readily available olefin 13, followed by isolation of the target isomers using crystallization and distillation. This process employed three parallel batches with an average yield of 39%, yielding 23 g of trans-16'.
Figure 4. Alternative Cyclobutane Building Block—Cyano Epoxide trans-16'
Preparation of Intermediate 11' is also essential for the synthesis of EBL-3183. Intermediate 11' can be obtained in large quantities (up to 160 g in a single batch) by cyclization. The process was accomplished using Suzuki-Miyaura cross-coupling reaction.
Figure 5.Synthesis of Building Block trans-16′ and Intermediate 11′
In addition, the nitrile reduction step was successfully implemented, confirming 11' as a suitable molecular building block for the synthesis of alternate C3 analogs. Scalable refinement by pH-controlled precipitation allowed the overall purity of the target compound 2 (EBL-3183) to be increased to an adequate level of 95.3-95.5%, which fulfills the need for high purity in preclinical studies.
Summarize
Overall, the synthetic route led to the preparation of the chiral structural molecular building block 9 in a total yield of 47% after six key steps using indole 3 as the starting material. The highly stereoselective cyclobutane structural unit trans-16′ was prepared by successful isolation of the target isomer by means of crystallization and distillation. Finally, a novel MBL inhibitor candidate (EBL-3183) was obtained in a total yield of 29% through five steps, which satisfied the requirements of sufficient scale and purity.
Reference
1.Andrei Baran,Jevgenijs Kuzmins. et al. Optimized Synthesis of Indole Carboxylate Metallo-β-Lactamase Inhibitor EBL-3183. Org. Process Res. Dev. 2023, 27, 4, 692–706. https://doi.org/10.1021/acs.oprd.3c00002
Aladdin:https://www.aladdinsci.com