Determine the necessary mass, volume, or concentration for preparing a solution.
Basic Description
| Specifications & Purity | sufficient for 100colorimetrictests |
|---|---|
| Storage Temp | Store at -20°C |
| Shipped In | Ice chest + Ice pads |
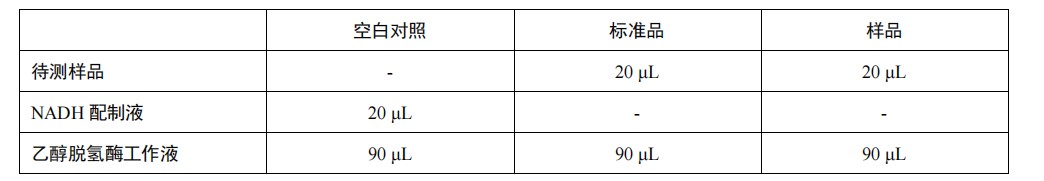
| Product Description | Nicotinamide adenine dinucleotide ( NAD ) is a coenzyme present in all cells, including NAD + ( oxidized ) and NADH ( reduced ) forms. NAD + is not only a coenzyme that transfers electrons during redox reactions, but also can be used as a substrate for many enzymes to participate in intracellular reactions. NAD + plays an important role in cells and in vivo. Its synthesis and degradation and its products are involved in apoptosis, metabolic regulation and gene expression regulation, and the reduction of NAD + is one of the main factors of cell death. The importance of NAD + in regulating the redox state of cells and the function of regulating signaling pathways and transcription make NAD + and the enzymes it synthesizes and consumes become potential drug targets for many diseases.The traditional determination of NAD + / NADH and NADP + / NADPH is accomplished by detecting the absorption at 340 nm. This method has low sensitivity and is susceptible to interference. NAD / NADH detection kit is a color reaction based on WST-8, by colorimetric method to detect cells, tissues or other samples of NAD + ( oxidized coenzyme I ) and NADH ( reduced coenzyme I ) respective amount, ratio and total amount of detection kit. The NAD / NADH detection kit can specifically detect NAD + and NADH, but not NADP + and NADPH. During the reaction, NAD + is reduced to NADH, and NADH reduces WST-8 to orange formazan, with a maximum absorption peak at about 450 nm. The formazan formed in the reaction system was proportional to the total amount of NAD + or NADH in the sample. The kit can detect 0.1 μM-10 μM NAD + or NADH. Composition: Matters needing attention: 1.It is recommended that the NADH standard should be dissolved and stored in small quantities to avoid repeated freezing and thawing ; 2.the NAD + / NADH extract is sticky, and it is necessary to ensure that it is fully mixed with the system to be added during use ; 3.In the process of sample addition and mixing, bubbles should be avoided to affect the final absorbance detection ; 4.Because NAD + / NADH is unstable and easy to degrade, so try to use fresh samples for detection ; 5.For your safety and health, please wear experimental clothes and disposable gloves. Usage: |
Certificates
Certificate of Analysis(COA)
Enter Lot Number to search for COA:
Find and download the COA for your product by matching the lot number on the packaging.
| Lot Number | Certificate Type | Date | Item |
|---|---|---|---|
| Certificate of Analysis | Dec 16, 2023 | N486176 |
Related Documents
Citations of This Product
| 1. Yu-Gang Shi,Run-Run Zhang,Chen-Min Zhu,Ming-Feng Xu,Qing Gu,Rammile Ettelaie,Shan Lin,Yi-Fan Wang,Xin-Yi Leng. (2021-01-09) Antimicrobial mechanism of alkyl gallates against Escherichia coli and Staphylococcus aureus and its combined effect with electrospun nanofibers on Chinese Taihu icefish preservation.. Food chemistry, 346 (128949-128949). [PMID:33418419] |
References
| 1. Eirini Pantazi,Emma Folch-Puy,Mohamed Bejaoui,Arnau Panisello,Ana Teresa Varela,Anabela Pinto Rolo,Carlos Marques Palmeira,Joan Roselló-Catafau. (2015-11-06) PPARα Agonist WY-14643 Induces SIRT1 Activity in Rat Fatty Liver Ischemia-Reperfusion Injury.. BioMed research international, 2015 (894679-894679). [PMID:26539534] |
| 2. Xingyin Liu,Julie Secombe. (2015-12-18) The Histone Demethylase KDM5 Activates Gene Expression by Recognizing Chromatin Context through Its PHD Reader Motif.. Cell reports, 13 ((10)): (2219-2231). [PMID:26673323] |
| 3. Xiu-Ming Wu,Chang-Qing Yang,Ying-Bo Mao,Ling-Jian Wang,Xiao-Xia Shangguan,Xiao-Ya Chen. (2016-02-26) Targeting insect mitochondrial complex I for plant protection.. Plant biotechnology journal, 14 ((9)): (1925-1935). [PMID:26914579] |
| 4. Violeta I Gallardo-Montejano,Geetu Saxena,Christine M Kusminski,Chaofeng Yang,John L McAfee,Lisa Hahner,Kathleen Hoch,William Dubinsky,Vihang A Narkar,Perry E Bickel. (2016-08-25) Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1α/SIRT1-dependent transcriptional regulation of mitochondrial function.. Nature communications, 7 (12723-12723). [PMID:27554864] |
| 5. Anders E Berglund,Kristen E N Scott,Weimin Li,Chunying Yang,Mario R Fernandez,Franz X Schaub,John L Cleveland,Robert J Rounbehler. (2016-11-09) Tristetraprolin disables prostate cancer maintenance by impairing proliferation and metabolic function.. Oncotarget, 7 ((50)): (83462-83475). [PMID:27825143] |
| 6. Alessia Angelin,Luis Gil-de-Gómez,Satinder Dahiya,Jing Jiao,Lili Guo,Matthew H Levine,Zhonglin Wang,William J Quinn,Piotr K Kopinski,Liqing Wang,Tatiana Akimova,Yujie Liu,Tricia R Bhatti,Rongxiang Han,Benjamin L Laskin,Joseph A Baur,Ian A Blair,Douglas C Wallace,Wayne W Hancock,Ulf H Beier. (2017-04-19) Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments.. Cell metabolism, 25 ((6)): (1282-1293). [PMID:28416194] |
| 7. Deepanwita Banerjee,Dharmeshkumar Parmar,Nivedita Bhattacharya,Avinash D Ghanate,Venkateswarlu Panchagnula,Anu Raghunathan. (2017-04-28) A scalable metabolite supplementation strategy against antibiotic resistant pathogen Chromobacterium violaceum induced by NAD. BMC systems biology, 11 ((1)): (51-51). [PMID:28446174] |
| 8. Brittany Kiehler,Lindsey Haggett,Masaya Fujita. (2017-04-28) The PAS domains of the major sporulation kinase in Bacillus subtilis play a role in tetramer formation that is essential for the\xa0autokinase activity.. MicrobiologyOpen, 6 ((4)): [PMID:28449380] |
| 9. Gabriela Segerer,Daria Engelmann,Alexandra Kaestner,Martin Trötzmüller,Harald Köfeler,Christian Stigloher,Christoph Thiele,Elisabeth Jeanclos,Antje Gohla. (2018-03-11) A phosphoglycolate phosphatase/AUM-dependent link between triacylglycerol turnover and epidermal growth factor signaling.. Biochimica et biophysica acta. Molecular and cell biology of lipids, 1863 ((6)): (584-594). [PMID:29524543] |
| 10. Zaijun Ma,Hui Wang,Yuping Cai,Han Wang,Kongyan Niu,Xiaofen Wu,Huanhuan Ma,Yun Yang,Wenhua Tong,Feng Liu,Zhandong Liu,Yaoyang Zhang,Rui Liu,Zheng-Jiang Zhu,Nan Liu. (2018-05-29) Epigenetic drift of H3K27me3 in aging links glycolysis to healthy longevity in Drosophila.. eLife, 7 [PMID:29809154] |
| 11. Kaitlyn E Wong,Maria C Mora,Nazneen Sultana,Kevin P Moriarty,Richard B Arenas,Nagendra Yadava,Sallie S Schneider,Michael V Tirabassi. (2018-06-07) Evaluation of Rhodiola crenulata on growth and metabolism of NB-1691, an MYCN-amplified neuroblastoma cell line.. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine, 40 ((6)): (1010428318779515-1010428318779515). [PMID:29871587] |
| 12. Andrea M Lencina,Thierry Franza,Matthew J Sullivan,Glen C Ulett,Deepak S Ipe,Philippe Gaudu,Robert B Gennis,Lici A Schurig-Briccio. (2018-07-05) Type 2 NADH Dehydrogenase Is the Only Point of Entry for Electrons into the Streptococcus agalactiae Respiratory Chain and Is a Potential Drug Target.. mBio, 9 ((4)): [PMID:29970468] |
| 13. Thomas Bertero,William M Oldham,Eloise M Grasset,Isabelle Bourget,Etienne Boulter,Sabrina Pisano,Paul Hofman,Floriant Bellvert,Guerrino Meneguzzi,Dmitry V Bulavin,Soline Estrach,Chloe C Feral,Stephen Y Chan,Alexandre Bozec,Cedric Gaggioli. (2018-10-09) Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy.. Cell metabolism, 29 ((1)): (124-140). [PMID:30293773] |
| 14. T O Ajiboye,E Skiebe,G Wilharm. (2018-12-12) Impact of zinc uptake regulator Zur on the susceptibility and oxidative stress response of Acinetobacter baumannii to antibiotics.. International journal of antimicrobial agents, 53 ((4)): (467-473). [PMID:30528772] |
| 15. Ji Heon Noh,Kyoung Mi Kim,Poonam R Pandey,Nicole Noren Hooten,Rachel Munk,Gautam Kundu,Supriyo De,Jennifer L Martindale,Xiaoling Yang,Michele K Evans,Kotb Abdelmohsen,Myriam Gorospe. (2019-02-13) Loss of RNA-binding protein GRSF1 activates mTOR to elicit a proinflammatory transcriptional program.. Nucleic acids research, 47 ((5)): (2472-2486). [PMID:30753671] |
| 16. Varsha Patil,Vikas Jain. (2019-07-10) Insights into the Physiology and Metabolism of a Mycobacterial Cell in an Energy-Compromised State.. Journal of bacteriology, 201 ((19)): [PMID:31285242] |
| 17. Diana Bahhir,Cagri Yalgin,Liina Ots,Sampsa Järvinen,Jack George,Alba Naudí,Tatjana Krama,Indrikis Krams,Mairi Tamm,Ana Andjelković,Eric Dufour,Jose M González de Cózar,Mike Gerards,Mikael Parhiala,Reinald Pamplona,Howard T Jacobs,Priit Jõers. (2019-10-05) Manipulating mtDNA in vivo reprograms metabolism via novel response mechanisms.. PLoS genetics, 15 ((10)): (e1008410-e1008410). [PMID:31584940] |
| 18. Nobu Oshima,Ryo Ishida,Shun Kishimoto,Kristin Beebe,Jeffrey R Brender,Kazutoshi Yamamoto,Daniel Urban,Ganesha Rai,Michelle S Johnson,Gloria Benavides,Giuseppe L Squadrito,Dan Crooks,Joseph Jackson,Abhinav Joshi,Bryan T Mott,Jonathan H Shrimp,Michael A Moses,Min-Jung Lee,Akira Yuno,Tobie D Lee,Xin Hu,Tamara Anderson,Donna Kusewitt,Helen H Hathaway,Ajit Jadhav,Didier Picard,Jane B Trepel,James B Mitchell,Gordon M Stott,William Moore,Anton Simeonov,Larry A Sklar,Jeffrey P Norenberg,W Marston Linehan,David J Maloney,Chi V Dang,Alex G Waterson,Matthew Hall,Victor M Darley-Usmar,Murali C Krishna,Leonard M Neckers. (2020-02-13) Dynamic Imaging of LDH Inhibition in Tumors Reveals Rapid In\xa0Vivo Metabolic Rewiring and Vulnerability to Combination Therapy.. Cell reports, 30 ((6)): (1798-1810). [PMID:32049011] |
| 19. Haiping Wang,Fabien Franco,Yao-Chen Tsui,Xin Xie,Marcel P Trefny,Roberta Zappasodi,Syed Raza Mohmood,Juan Fernández-García,Chin-Hsien Tsai,Isabell Schulze,Florence Picard,Etienne Meylan,Roy Silverstein,Ira Goldberg,Sarah-Maria Fendt,Jedd D Wolchok,Taha Merghoub,Camilla Jandus,Alfred Zippelius,Ping-Chih Ho. (2020-02-19) CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors.. Nature immunology, 21 ((3)): (298-308). [PMID:32066953] |
| 20. Francois Brial,Fawaz Alzaid,Kazuhiro Sonomura,Yoichiro Kamatani,Kelly Meneyrol,Aurélie Le Lay,Noémie Péan,Lyamine Hedjazi,Taka-Aki Sato,Nicolas Venteclef,Christophe Magnan,Mark Lathrop,Marc-Emmanuel Dumas,Fumihiko Matsuda,Pierre Zalloua,Dominique Gauguier. (2020-02-23) The Natural Metabolite 4-Cresol Improves Glucose Homeostasis and Enhances β-Cell Function.. Cell reports, 30 ((7)): (2306-2320). [PMID:32075738] |
| 21. Igor Shats,Jason G Williams,Juan Liu,Mikhail V Makarov,Xiaoyue Wu,Fred B Lih,Leesa J Deterding,Chaemin Lim,Xiaojiang Xu,Thomas A Randall,Ethan Lee,Wenling Li,Wei Fan,Jian-Liang Li,Marina Sokolsky,Alexander V Kabanov,Leping Li,Marie E Migaud,Jason W Locasale,Xiaoling Li. (2020-03-05) Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway.. Cell metabolism, 31 ((3)): (564-579). [PMID:32130883] |
| 22. Lici A Schurig-Briccio,Paola K Parraga Solorzano,Andrea M Lencina,Jana N Radin,Grischa Y Chen,John-Demian Sauer,Thomas E Kehl-Fie,Robert B Gennis. (2020-03-24) Role of respiratory NADH oxidation in the regulation of Staphylococcus aureus virulence.. EMBO reports, 21 ((5)): (e45832-e45832). [PMID:32202364] |
| 23. Victor Contreras-Jácquez,Jorge Rodríguez-González,Juan Carlos Mateos-Díaz,Elisa M Valenzuela-Soto,Ali Asaff-Torres. (2020-05-14) Differential Activation of Ferulic Acid Catabolic Pathways of Amycolatopsis sp. ATCC 39116 in Submerged and Surface Cultures.. Applied biochemistry and biotechnology, 192 ((2)): (494-516). [PMID:32399842] |
| 24. Anthony O Beas,Patricia B Gordon,Clara L Prentiss,Carissa Perez Olsen,Matthew A Kukurugya,Bryson D Bennett,Susan M Parkhurst,Daniel E Gottschling. (2020-06-05) Independent regulation of age associated fat accumulation and longevity.. Nature communications, 11 ((1)): (2790-2790). [PMID:32493904] |
| 25. Duo Feng,DongZhu Xu,Nobuyuki Murakoshi,Kazuko Tajiri,Rujie Qin,Saori Yonebayashi,Yuta Okabe,Siqi Li,Zixun Yuan,Kazutaka Aonuma,Masaki Ieda. (2020-07-08) Nicotinamide Phosphoribosyltransferase (Nampt)/Nicotinamide Adenine Dinucleotide (NAD) Axis Suppresses Atrial Fibrillation by Modulating the Calcium Handling Pathway.. International journal of molecular sciences, 21 ((13)): [PMID:32629939] |
| 26. Wonjae Seong,Gui Hwan Han,Hyun Seung Lim,Ji In Baek,Soo-Jung Kim,Donghyuk Kim,Seong Keun Kim,Hyewon Lee,Haseong Kim,Seung-Goo Lee,Dae-Hee Lee. (2020-09-16) Adaptive laboratory evolution of Escherichia coli lacking cellular byproduct formation for enhanced acetate utilization through compensatory ATP consumption.. Metabolic engineering, 62 (249-259). [PMID:32931907] |
| 27. Nicholas J Hunt,Glen P Lockwood,Sun W S Kang,Lara J Westwood,Christina Limantoro,Wojciech Chrzanowski,Peter A G McCourt,Zdenka Kuncic,David G Le Couteur,Victoria C Cogger. (2021-02-26) Quantum Dot Nanomedicine Formulations Dramatically Improve Pharmacological Properties and Alter Uptake Pathways of Metformin and Nicotinamide Mononucleotide in Aging Mice.. ACS nano, 15 ((3)): (4710-4727). [PMID:33626869] |
| 28. Poushali Chakraborty,Sapna Bajeli,Deepak Kaushal,Bishan Dass Radotra,Ashwani Kumar. (2021-03-13) Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis.. Nature communications, 12 ((1)): (1606-1606). [PMID:33707445] |
| 29. Ho Shing Chan,Kemeng Xiao,Tsz Ho Tsang,Cuiping Zeng,Bo Wang,Xingxing Peng,Po Keung Wong. (2021-05-11) Bioremediation of Crude Glycerol by a Sustainable Organic-Microbe Hybrid System.. Frontiers in microbiology, 12 (654033-654033). [PMID:33967990] |
| 30. Nzinga Mack,Elizabeth Mazzio,Ramesh Badisa,Karam F A Soliman. (2021-05-18) Metabolic Response to the Mitochondrial Toxin 1-Methyl-4-phenylpyridinium (MPP+) in LDH-A/B Double-knockout LS174T Colon Cancer Cells.. Cancer genomics & proteomics, 18 ((3 Suppl)): (385-405). [PMID:33994363] |
| 31. Jing Wu,Wei Li,Shi-Guang Zhao,Sen-He Qian,Zhou Wang,Meng-Jie Zhou,Wen-Song Hu,Jian Wang,Liu-Xiu Hu,Yan Liu,Zheng-Lian Xue. (2021-06-09) Site-directed mutagenesis of the quorum-sensing transcriptional regulator SinR affects\xa0the biosynthesis of menaquinone in\xa0Bacillus subtilis.. Microbial cell factories, 20 ((1)): (113-113). [PMID:34098969] |
| 32. Wei Li,Minghao Gong,Yuk Pheel Park,Ahmed S Elshikha,Seung-Chul Choi,Josephine Brown,Nathalie Kanda,Wen-I Yeh,Leeana Peters,Anton A Titov,Xiangyu Teng,Todd M Brusko,Laurence Morel. (2021-06-23) Lupus susceptibility gene Esrrg modulates regulatory T cells through mitochondrial metabolism.. JCI insight, 6 ((14)): [PMID:34156979] |
| 33. Hao Zhang,Xiaoyun Liu,Shengnan Ren,Mabrouk Elsabagh,Mengzhi Wang,Hongrong Wang. (2021-09-02) Dietary N-carbamylglutamate or l-arginine supplementation improves hepatic energy status and mitochondrial function and inhibits the AMP-activated protein kinase-peroxisome proliferator-activated receptor γ coactivator-1α-transcription factor A pathway in intrauterine-growth-retarded suckling lambs.. Animal nutrition (Zhongguo xu mu shou yi xue hui), 7 ((3)): (859-867). [PMID:34466690] |
| 34. Ting La,Song Chen,Tao Guo,Xiao Hong Zhao,Liu Teng,Dandan Li,Michael Carnell,Yuan Yuan Zhang,Yu Chen Feng,Nicole Cole,Alexandra C Brown,Didi Zhang,Qihan Dong,Jenny Y Wang,Huixia Cao,Tao Liu,Rick F Thorne,Feng-Min Shao,Xu Dong Zhang,Lei Jin. (2021-10-15) Visualization of endogenous p27 and Ki67 reveals the importance of a c-Myc-driven metabolic switch in promoting survival of quiescent cancer cells.. Theranostics, 11 ((19)): (9605-9622). [PMID:34646389] |
| 35. Pengyi Yan,Zixuan Li,Junhao Xiong,Zilong Geng,Weiting Wei,Yan Zhang,Gengze Wu,Tao Zhuang,Xiaoyu Tian,Zhijie Liu,Junling Liu,Kun Sun,Fengyuan Chen,Yuzhen Zhang,Chunyu Zeng,Yu Huang,Bing Zhang. (2021-11-25) LARP7 ameliorates cellular senescence and aging by allosterically enhancing SIRT1 deacetylase activity.. Cell reports, 37 ((8)): (110038-110038). [PMID:34818543] |
| 36. Frédérique Moyrand,Ingrid Lafontaine,Thierry Fontaine,Guilhem Janbon. (2008-09-30) UGE1 and UGE2 regulate the UDP-glucose/UDP-galactose equilibrium in Cryptococcus neoformans.. Eukaryotic cell, 7 ((12)): (2069-2077). [PMID:18820075] |
| 37. Eirini Pantazi,Mohamed Amine Zaouali,Mohamed Bejaoui,Emma Folch-Puy,Hassen Ben Abdennebi,Ana Teresa Varela,Anabela Pinto Rolo,Carlos Marques Palmeira,Joan Roselló-Catafau. (2015-02-17) Sirtuin 1 in rat orthotopic liver transplantation: an IGL-1 preservation solution approach.. World journal of gastroenterology, 21 ((6)): (1765-1774). [PMID:25684941] |
| 38. Shaimaa Ahmed,Debbie Bott,Alvin Gomez,Laura Tamblyn,Adil Rasheed,Tiffany Cho,Laura MacPherson,Kim S Sugamori,Yang Yang,Denis M Grant,Carolyn L Cummins,Jason Matthews. (2015-05-16) Loss of the Mono-ADP-ribosyltransferase, Tiparp, Increases Sensitivity to Dioxin-induced Steatohepatitis and Lethality.. The Journal of biological chemistry, 290 ((27)): (16824-16840). [PMID:25975270] |
| 39. Taofeek O Ajiboye,Ramat S Habibu,Kabiru Saidu,Fatimah Z Haliru,Hikmat O Ajiboye,Najeeb O Aliyu,Oluwayemisi B Ibitoye,Judith N Uwazie,Hamdalat F Muritala,Sharafa A Bello,Idris I Yusuf,Aisha O Mohammed. (2017-03-30) Involvement of oxidative stress in protocatechuic acid-mediated bacterial lethality.. MicrobiologyOpen, 6 ((4)): [PMID:28349673] |
| 40. Patrick T Kang,Chwen-Lih Chen,Paul Lin,William M Chilian,Yeong-Renn Chen. (2017-05-17) Impairment of pH gradient and membrane potential mediates redox dysfunction in the mitochondria of the post-ischemic heart.. Basic research in cardiology, 112 ((4)): (36-36). [PMID:28508960] |
| 41. Nima Borhan Fakouri,Jon Ambæk Durhuus,Christine Elisabeth Regnell,Maria Angleys,Claus Desler,Md Mahdi Hasan-Olive,Ana Martín-Pardillos,Anastasia Tsaalbi-Shtylik,Kirsten Thomsen,Martin Lauritzen,Vilhelm A Bohr,Niels de Wind,Linda Hildegard Bergersen,Lene Juel Rasmussen. (2017-10-04) Rev1 contributes to proper mitochondrial function via the PARP-NAD. Scientific reports, 7 ((1)): (12480-12480). [PMID:28970491] |
| 42. Junjun Wu,Xia Zhang,Peng Zhou,Jiaying Huang,Xiudong Xia,Wei Li,Ziyu Zhou,Yue Chen,Yinghao Liu,Mingsheng Dong. (2017-11-11) Improving metabolic efficiency of the reverse beta-oxidation cycle by balancing redox cofactor requirement.. Metabolic engineering, 44 (313-324). [PMID:29122703] |
| 43. Mandeep Kaur,Guhan Jayaraman. (2016-01-22) Hyaluronan production and molecular weight is enhanced in pathway-engineered strains of lactate dehydrogenase-deficient Lactococcus lactis.. Metabolic engineering communications, 3 (15-23). [PMID:29468110] |
| 44. Lane C Porter,Michael P Franczyk,Terri Pietka,Shintaro Yamaguchi,Jonathan B Lin,Yo Sasaki,Eric Verdin,Rajendra S Apte,Jun Yoshino. (2018-04-11) NAD+-dependent deacetylase SIRT3 in adipocytes is dispensable for maintaining normal adipose tissue mitochondrial function and whole body metabolism.. American journal of physiology. Endocrinology and metabolism, 315 ((4)): (E520-E530). [PMID:29634313] |
| 45. Kumar Subramani,Xiaogang Chu,Marie Warren,Mariah Lee,Sumin Lu,Nagendra Singh,Raghavan Raju. (2019-01-10) Deficiency of metabolite sensing receptor HCA2 impairs the salutary effect of niacin in hemorrhagic shock.. Biochimica et biophysica acta. Molecular basis of disease, 1865 ((3)): (688-695). [PMID:30625381] |
| 46. Wei-He Qian,Yuan-Yuan Liu,Xiang Li,Yan Pan. (2019-06-08) MicroRNA-141 ameliorates alcoholic hepatitis‑induced intestinal injury and intestinal endotoxemia partially via a TLR4-dependent mechanism.. International journal of molecular medicine, 44 ((2)): (569-581). [PMID:31173169] |
| 47. Chenyang Ye,Lina Qi,Xiaofen Li,Ji Wang,Jiekai Yu,Biting Zhou,Cheng Guo,Jiani Chen,Shu Zheng. (2020-02-02) Targeting the NAD+ salvage pathway suppresses APC mutation-driven colorectal cancer growth and Wnt/β-catenin signaling via increasing Axin level.. Cell communication and signaling : CCS, 18 ((1)): (16-16). [PMID:32005247] |
| 48. Esther P Jane,Daniel R Premkumar,Swetha Thambireddy,Brian Golbourn,Sameer Agnihotri,Kelsey C Bertrand,Stephen C Mack,Max I Myers,Ansuman Chattopadhyay,D Lansing Taylor,Mark E Schurdak,Andrew M Stern,Ian F Pollack. (2020-04-03) Targeting NAD+ Biosynthesis Overcomes Panobinostat and Bortezomib-Induced Malignant Glioma Resistance.. Molecular cancer research : MCR, 18 ((7)): (1004-1017). [PMID:32238439] |
| 49. Hao Gu,Chiqi Chen,Xiaoxin Hao,Ni Su,Dan Huang,Yejun Zou,Shu-Hai Lin,Xianjun Chen,Denghao Zheng,Ligen Liu,Zhuo Yu,Li Xie,Yaping Zhang,Xiaoxiao He,Xiaoyun Lai,Xiaocui Zhang,Guo-Qiang Chen,Yuzheng Zhao,Yi Yang,Joseph Loscalzo,Junke Zheng. (2020-05-13) MDH1-mediated malate-aspartate NADH shuttle maintains the activity levels of fetal liver hematopoietic stem cells.. Blood, 136 ((5)): (553-571). [PMID:32396938] |
| 50. Yu-Gang Shi,Run-Run Zhang,Chen-Min Zhu,Ming-Feng Xu,Qing Gu,Rammile Ettelaie,Shan Lin,Yi-Fan Wang,Xin-Yi Leng. (2021-01-09) Antimicrobial mechanism of alkyl gallates against Escherichia coli and Staphylococcus aureus and its combined effect with electrospun nanofibers on Chinese Taihu icefish preservation.. Food chemistry, 346 (128949-128949). [PMID:33418419] |
| 51. Yilong Miao,Xinyu Li,Xiaoyan Shi,Qian Gao,Jingyue Chen,Rui Wang,Yong Fan,Bo Xiong. (2021-02-20) Nicotinamide Mononucleotide Restores the Meiotic Competency of Porcine Oocytes Exposed to Ethylene Glycol Butyl Ether.. Frontiers in cell and developmental biology, 9 (628580-628580). [PMID:33604339] |
| 52. Wencui Wan,Wei Zhu,Yan Wu,Yang Long,Hongzhuo Liu,Weiwei Wan,Guangming Wan,Jing Yu. (2021-07-22) Grape Seed Proanthocyanidin Extract Moderated Retinal Pigment Epithelium Cellular Senescence Through NAMPT/SIRT1/NLRP3 Pathway.. Journal of inflammation research, 14 (3129-3143). [PMID:34285539] |