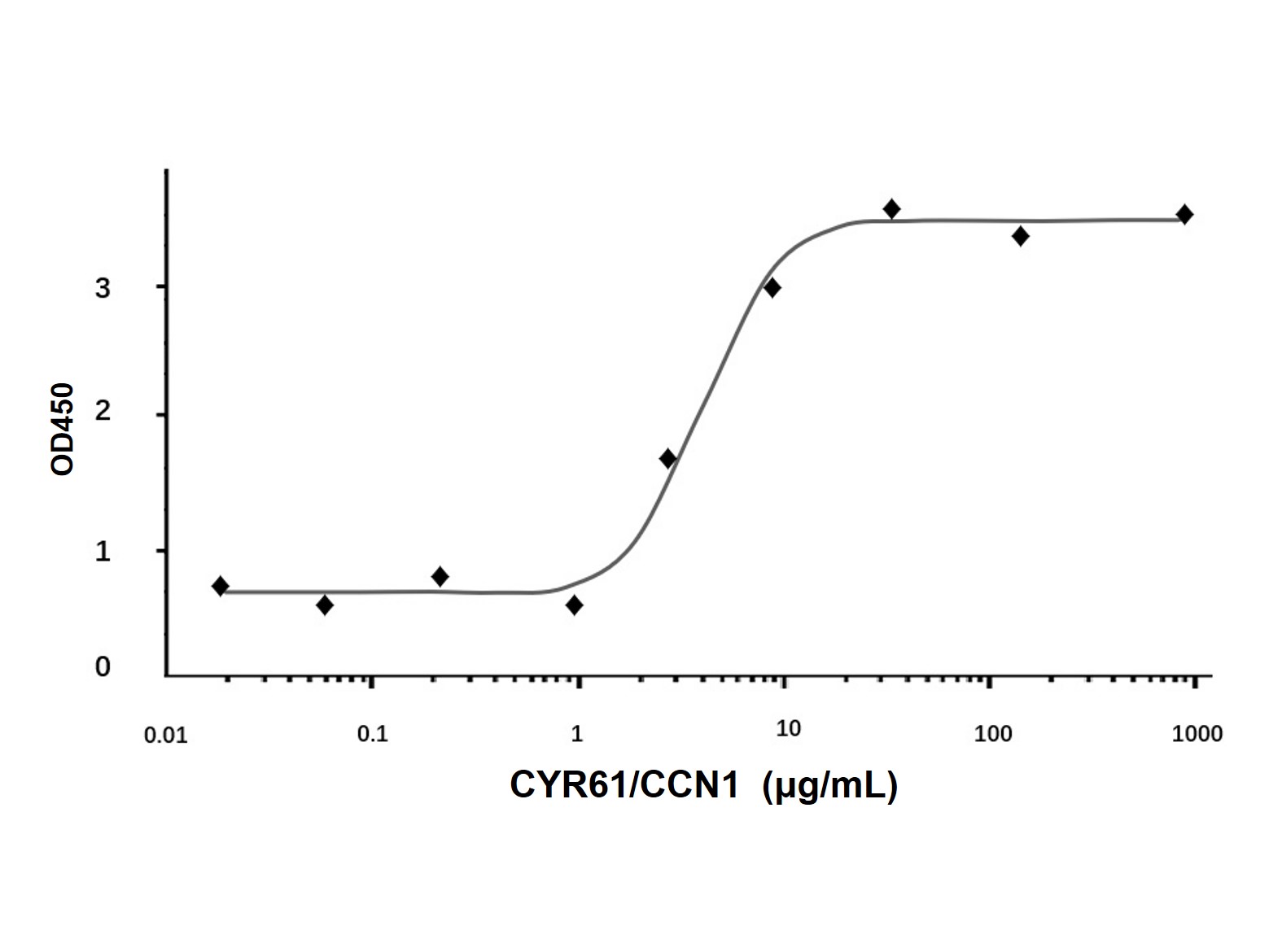
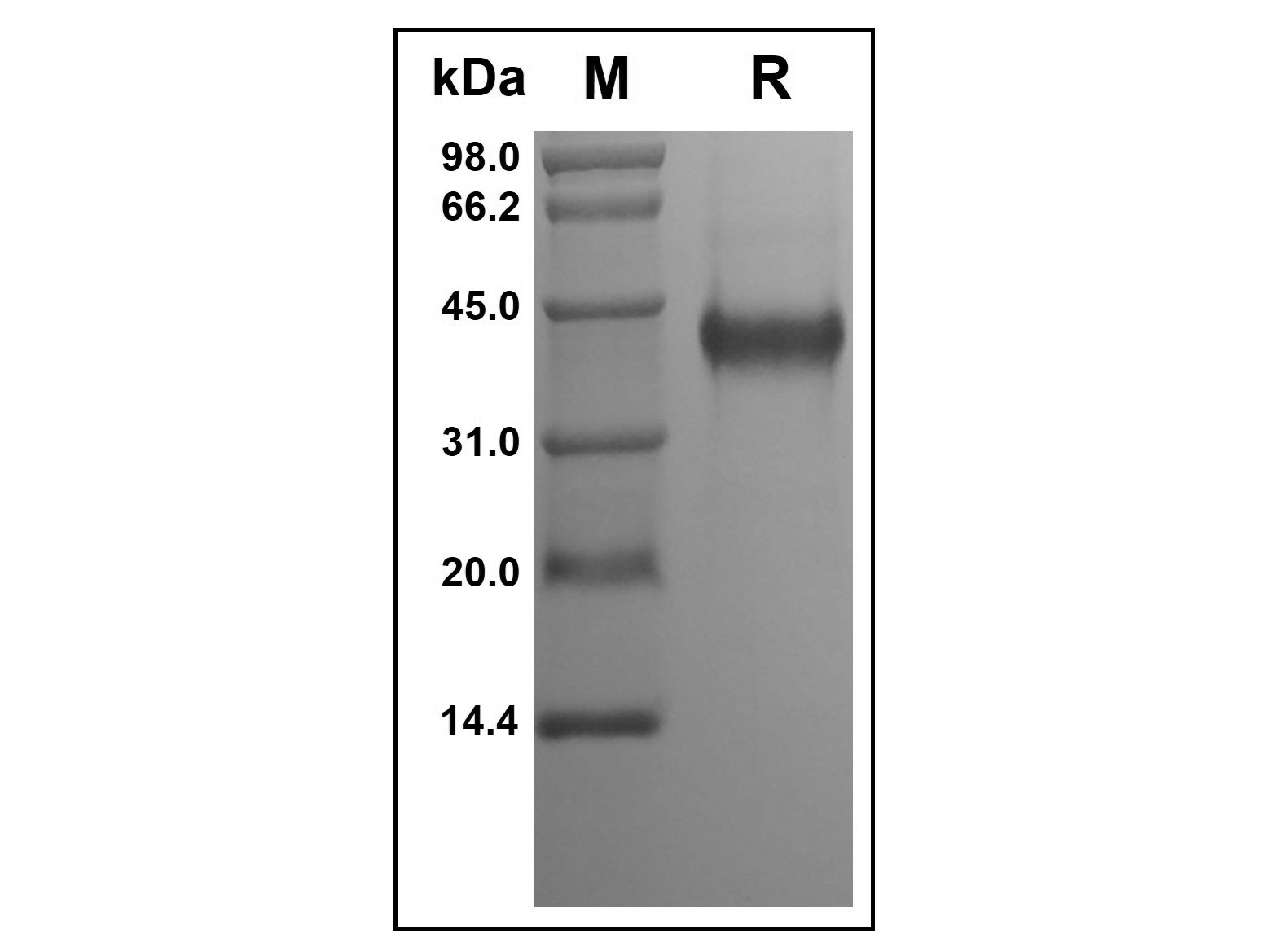
| Product Description | Purity
> 95 % by SDS-PAGE and HPLC analyses.
Function
Promotes cell proliferation, chemotaxis, angiogenesis and cell adhesion. Appears to play a role in wound healing by up-regulating, in skin fibroblasts, the expression of a number of genes involved in angiogenesis, inflammation and matrix remodeling including VEGA-A, VEGA-C, MMP1, MMP3, TIMP1, uPA, PAI-1 and integrins alpha-3 and alpha-5. CYR61-mediated gene regulation is dependent on heparin-binding. Down-regulates the expression of alpha-1 and alpha-2 subunits of collagen type-1. Promotes cell adhesion and adhesive signaling through integrin alpha-6/beta-1, cell migration through integrin alpha-v/beta-5 and cell proliferation through integrin alpha-v/beta-3.
Banckground:
Cyr61, also known as CCN1, is a 40-45 kDa matricellular glycoprotein that plays an important role in cellular adhesion and migration (1). Cyr61 consists of an IGFBP domain, a VWF type C domain, a TSP type I domain, and a cysteine knot domain (2). Mature human Cyr61 shares 93% amino acid sequence identity with mouse and rat Cyr61. It is widely expressed during development and in adult tissues (2, 3). Cyr61 associates with the extracellular matrix (ECM) and with many cell surface molecules including Integrins alpha V beta 3, alpha V beta 5, alpha M beta 2, and alpha 6 beta 1, Syndecan-4, and heparan sulfate proteoglycans (1, 3). Cyr61 mediates the adhesion and migration of multiple cell types and also promotes vascular endothelial cell tubule formation (4-6). Plasmin cleavage of ECM-bound Cyr61 releases a 28 kDa N-terminal fragment which retains the ability to promote endothelial cell migration (7). Cyr61 exhibits both tumorigenic and tumor suppressor properties. It is up-regulated and promotes tumorigenesis, angiogenesis, and metastasis in breast, renal, gastric, squamous cell, and colorectal carcinomas as well as in glioma (8-12). In contrast, when
down-regulated, it suppresses tumor growth in endometrial, hepatic, and non-small cell lung cancers (8, 13, 14). Cyr61 is also up-regulated in injured skin and bone where it induces the expression of growth factors, cytokines, proteases, and integrins involved in wound repair (15, 16).
|
|---|