
Recombinant Human EGF Protein (rp145392)-Protein Bioactivity
Measured in a cell proliferation assay using BALB/3T3 mouse embryo fibroblasts cell line. The ED₅₀ for this effect is typically <0.9ng/mL.
| SKU | Size | Availability | Price | Qty |
|---|---|---|---|---|
| rp145392-100μg (Trial Size) Apply for free trial size(?) Every year, as a valued customer, you have the exclusive opportunity to explore and enjoy three different trial products of your choice, absolutely free! | 100μg | Available within 4-8 weeks(?) Items will be manufactured post-order and can take 4-8 weeks. Thank you for your patience! | $168.90 | |
| rp145392-1mg | 1mg | Available within 4-8 weeks(?) Items will be manufactured post-order and can take 4-8 weeks. Thank you for your patience! | $554.90 | |
| rp145392-500μg | 500μg | Available within 4-8 weeks(?) Items will be manufactured post-order and can take 4-8 weeks. Thank you for your patience! | $334.90 |
GMP, >95% SDS-PAGE, Active,Pichia pastoris, No tag, 971-1021aa
| Product Name | Recombinant Human EGF Protein, >95% SDS-PAGE, high purity |
|---|---|
| Synonyms | beta-urogastrone; EGF; epidermal growth factor (beta-urogastrone); epidermal growth factor; hEGF; HOMG4; pro-epidermal growth factor; URG; Urogastrone |
| Specifications & Purity | ActiBioPure™, Bioactive, GMP, Carrier Free, Azide Free, High performance, ≥95%(SDS-PAGE) |
| Purity | >95% SDS-PAGE |
| Biological Activity | Measured in a cell proliferation assay using BALB/3T3 mouse embryo fibroblasts cell line. The ED₅₀ for this effect is typically <0.9 ng/mL. |
| Species | Human |
| Amino Acids | 971-1021 aa |
| Sequence | NSDSECPLSHDGYCLHDGVCMYIEALDKYACNCVVGYIGERCQYRDLKWW ELR |
| Protein Tag | No tag |
| N-terminal Sequence | NSDSECPLSH |
| Protein Length | Full length protein |
| Accession # | P01133 |
| Source | Recombinant |
| Predicted molecular weight | 6 kDa |
| Grade | ActiBioPure™, Azide Free, Bioactive, Carrier Free, GMP, High performance |

Recombinant Human EGF Protein (rp145392)-Protein Bioactivity
Measured in a cell proliferation assay using BALB/3T3 mouse embryo fibroblasts cell line. The ED₅₀ for this effect is typically <0.9ng/mL.
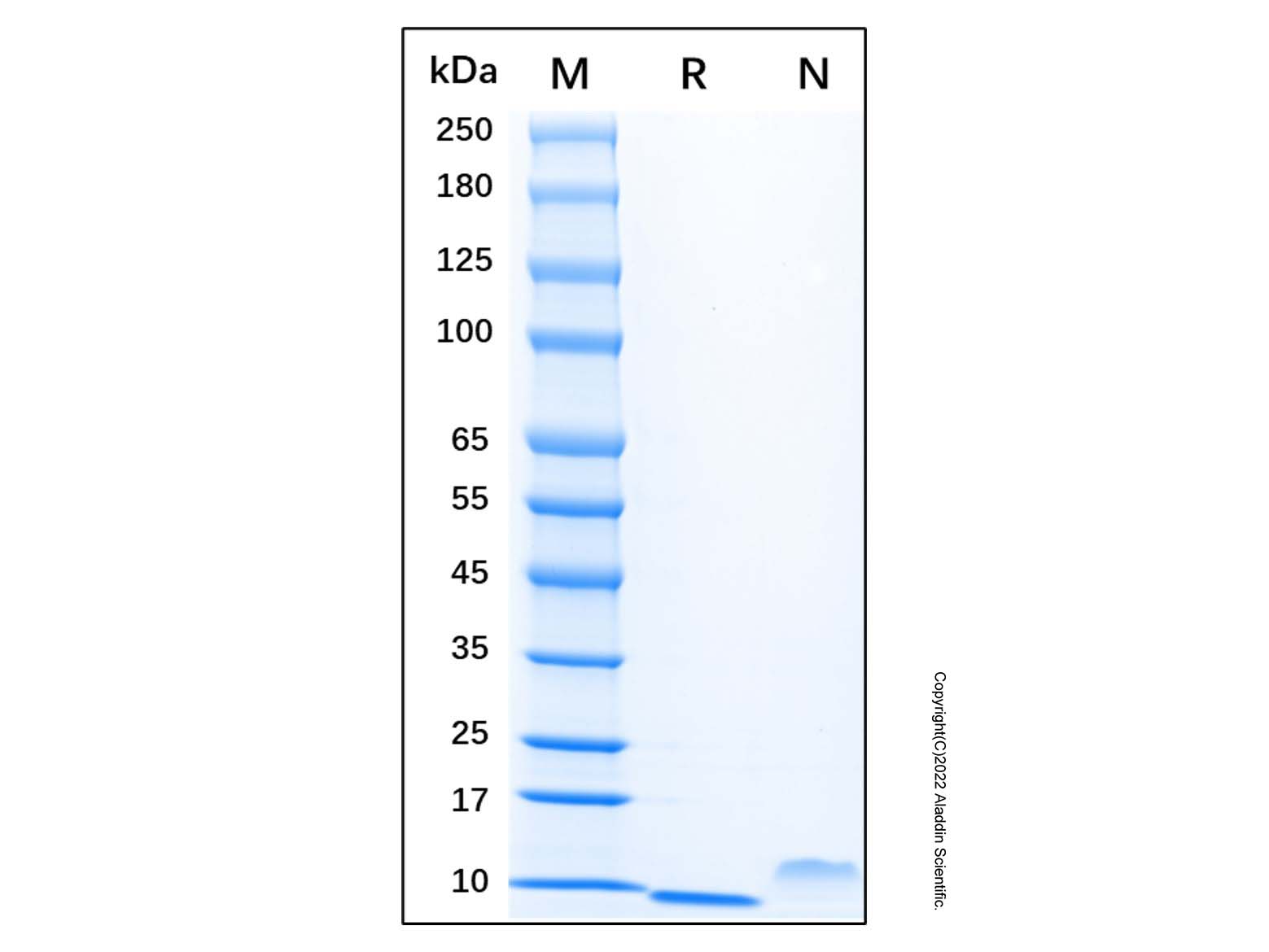
Recombinant Human EGF Protein (rp145392)-SDS-PAGE
5μg/lane of Recombinant Human EGF was resolved with SDS-PAGE under reducing (R) and non-reducing (N) conditions and visualized by Coomassie® Blue staining, showing a single band at 6.6 kDa.
| Form | Lyophilized |
|---|---|
| Reconstitution | We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in sterile 10mM PBS to a concentration of 0.1-1.0 mg/mL. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 |
| Storage Temp | Store at -20°C |
| Shipped In | Dry ice |
| Stability And Storage | Stability: stable at -20℃ to 24 months, stable at 2-8 ℃ to 4 weeks. Prepared as 100μg/ml storage solution, 2~8℃ can be stably stored for a week.Stability: stable at -20℃ to 24 months, stable at 2-8 ℃ to 4 weeks. Prepared as 100μg/ml storage solution, 2~8℃ |
Enter Lot Number to search for COA:
To view the certificate results,please click on a Lot number.For Lot numbers from past orders,please use our order status section
| Lot Number | Certificate Type | Date | Item |
|---|---|---|---|
| Certificate of Analysis | Apr 25, 2023 | rp145392 | |
| Certificate of Analysis | Apr 25, 2023 | rp145392 | |
| Certificate of Analysis | Apr 19, 2023 | rp145392 |
| 1. Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M et al.. (2002) Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains.. Cell, 110 (6): (775-87). [PMID:12297050] |
| 2. Feng Y, Yin Y, Weiser A, Griffin E, Cameron MD, Lin L, Ruiz C, Schürer SC, Inoue T, Rao PV, Schröter T, Lograsso P.. (2008) Discovery of substituted 4-(pyrazol-4-yl)-phenylbenzodioxane-2-carboxamides as potent and highly selective Rho kinase (ROCK-II) inhibitors.. J Med Chem, 51 (21): (6642-6645). [PMID:18834107] |
| 3. Lesuisse D, Mauger J, Nemecek C, Maignan S, Boiziau J, Harlow G, Hittinger A, Ruf S, Strobel H, Nair A, Ritter K, Malleron JL, Dagallier A, El-Ahmad Y, Guilloteau JP, Guizani H, Bouchard H, Venot C.. (2011) Discovery of the first non-ATP competitive IGF-1R kinase inhibitors: advantages in comparison with competitive inhibitors.. Bioorg Med Chem Lett, 21 (8): (2224-2228). [PMID:21441024] |
| 4. Hodgetts KJ, Ge P, Yoon T, De Lombaert S, Brodbeck R, Gulianello M, Kieltyka A, Horvath RF, Kehne JH, Krause JE, Maynard GD, Hoffman D, Lee Y, Fung L, Doller D.. (2011) Discovery of N-(1-ethylpropyl)-[3-methoxy-5-(2-methoxy-4-trifluoromethoxyphenyl)-6-methyl-pyrazin-2-yl]amine 59 (NGD 98-2): an orally active corticotropin releasing factor-1 (CRF-1) receptor antagonist.. J Med Chem, 54 (12): (4187-4206). [PMID:21618986] |
| 5. Gregory, H H and Preston, B M BM.. (1977) The primary structure of human urogastrone.. International journal of peptide and protein research, [PMID:300079] |
| 6. Hommel, U U, Harvey, T S TS, Driscoll, P C PC and Campbell, I D ID.. (1992) Human epidermal growth factor. High resolution solution structure and comparison with human transforming growth factor alpha.. Journal of molecular biology, (5): [PMID:1522591] |
| 7. Furuya, M M, Akashi, S S and Hirayama, K K.. (1989) The primary structure of human EGF produced by genetic engineering, studied by high-performance tandem mass spectrometry.. Biochemical and biophysical research communications, (15): [PMID:2789514] |
| 8. Bell, G I GI and 8 more authors.. (1986) Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization.. Nucleic acids research, (11): [PMID:3491360] |
| 9. Lu, H S HS and 5 more authors.. (2001) Crystal structure of human epidermal growth factor and its dimerization.. The Journal of biological chemistry, (14): [PMID:11438527] |
| 10. Ferguson, Kathryn M KM and 5 more authors.. (2003) EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization.. Molecular cell, [PMID:12620237] |
| 11. Hillier, Ladeana W LW and 121 more authors.. (2005) Generation and annotation of the DNA sequences of human chromosomes 2 and 4.. Nature, (7): [PMID:15815621] |
| 12. Groenestege, Wouter M Tiel WM and 10 more authors.. (2007) Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia.. The Journal of clinical investigation, [PMID:17671655] |
| 13. Lu, Chafen C and 6 more authors.. (2010) Structural evidence for loose linkage between ligand binding and kinase activation in the epidermal growth factor receptor.. Molecular and cellular biology, [PMID:20837704] |
| 14. Huang, Hsiao-Wen HW, Mohan, Sepuru K SK and Yu, C C.. (2010) The NMR solution structure of human epidermal growth factor (hEGF) at physiological pH and its interactions with suramin.. Biochemical and biophysical research communications, (26): [PMID:21029725] |
| 15. Zettl, Markus M, Adrain, Colin C, Strisovsky, Kvido K, Lastun, Viorica V and Freeman, Matthew M.. (2011) Rhomboid family pseudoproteases use the ER quality control machinery to regulate intercellular signaling.. Cell, (1): [PMID:21439629] |