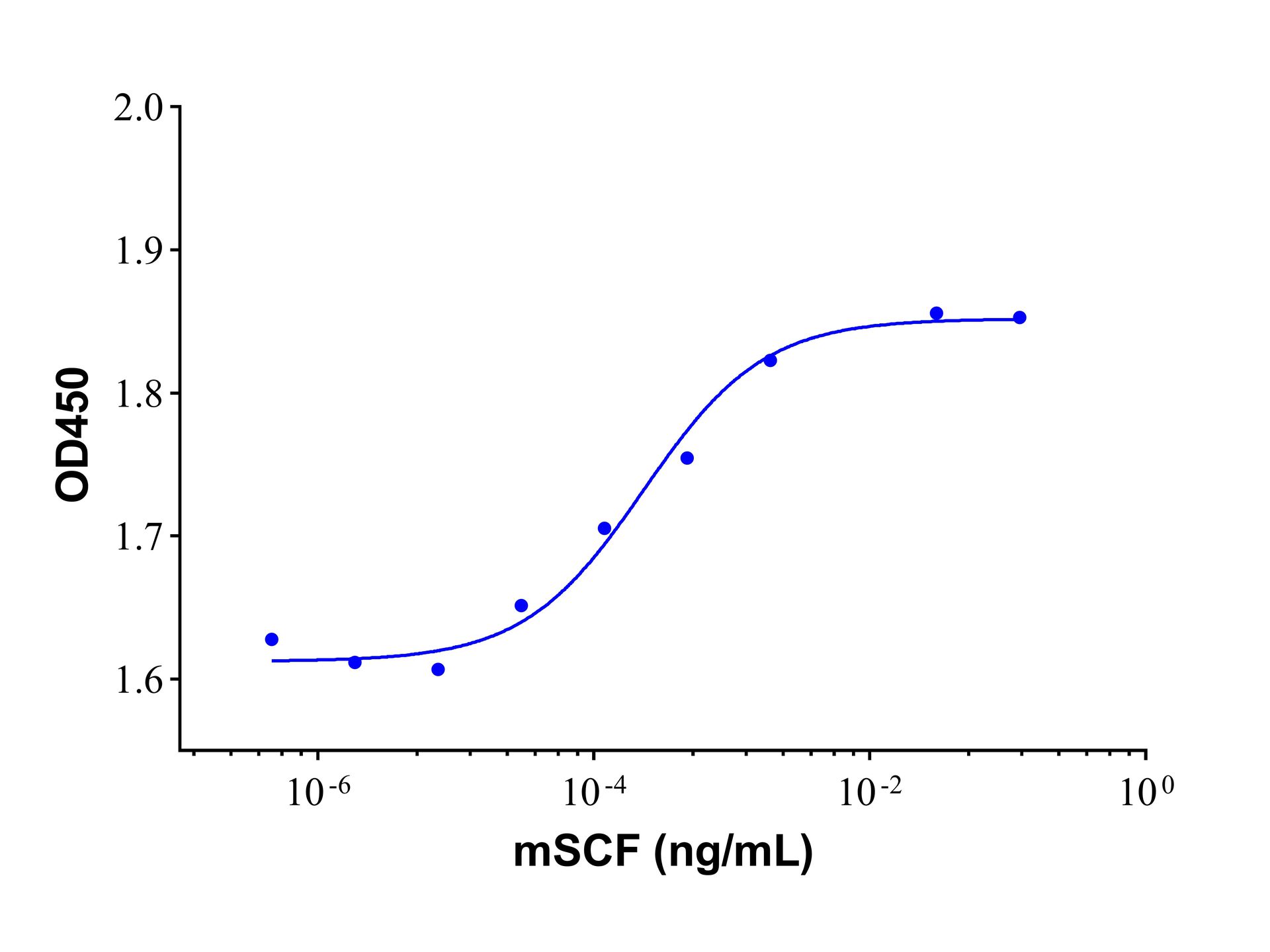
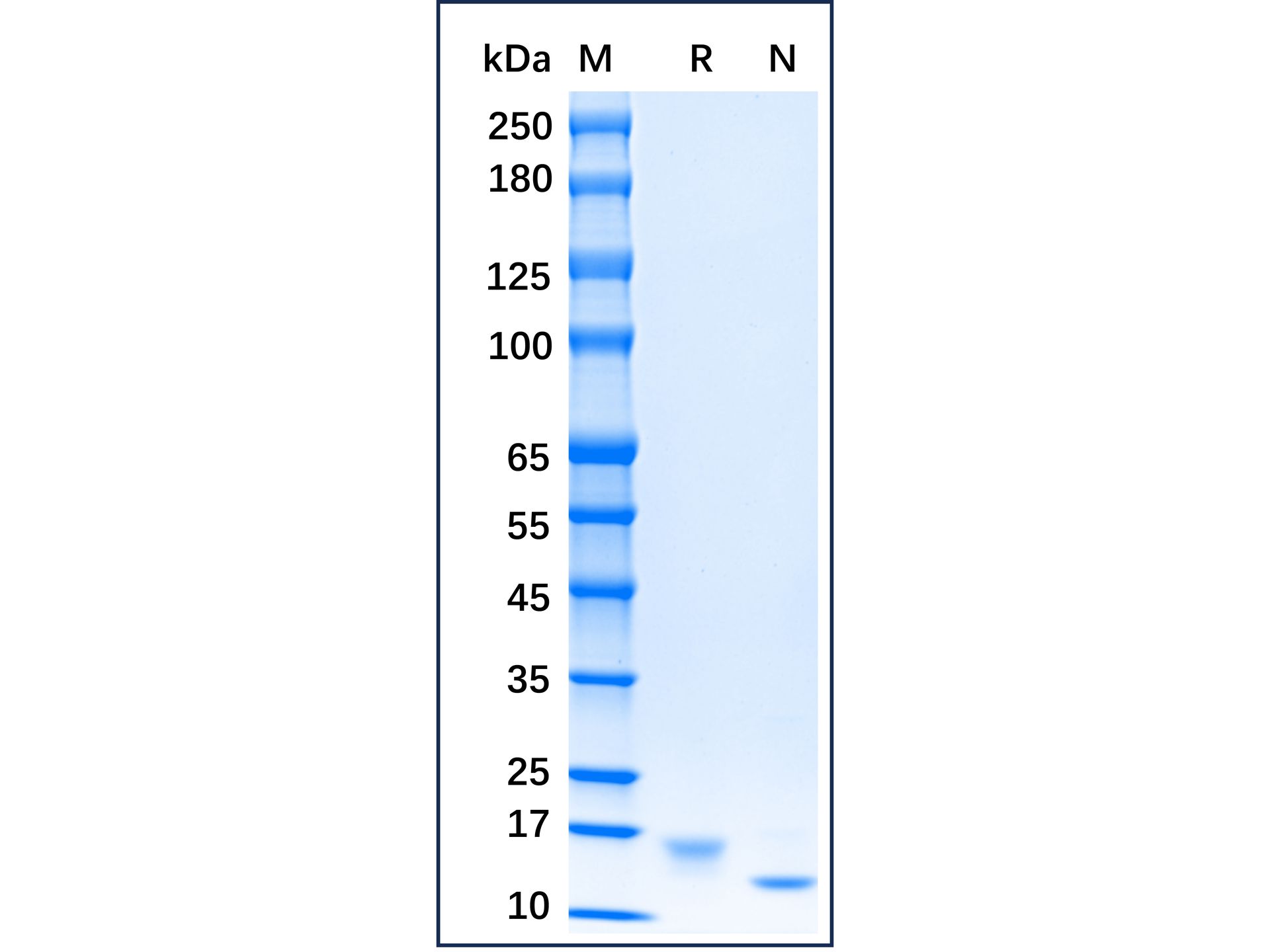
| Product Description | Stem Cell Factor (SCF) which binds to the c-Kit receptor is produced by fibroblasts and endothelial cells. The soluble and transmembrane forms of the protein are formed by alternative splicing of the same RNA transcript and the presence of both soluble and transmembrane It is required for normal hematopoietic function and plays an important role in hematopoiesis, spermatogenesis, and melanogenesis. It also promotes mast cell adhesion, migration, proliferation, and survival. Human SCF manifests low activity on murine cells, while murine and rat SCF are fully active on human cells. Recombinant murine SCF is an 18.4kDa polypeptide containing 165 amino acid residues.
Purity >97% (SDS-PAGE,HPLC)
Function Ligand for the receptor-type protein-tyrosine kinase KIT. Plays an essential role in the regulation of cell survival and proliferation, hematopoiesis, stem cell maintenance, gametogenesis, mast cell development, migration and function, and in melanogenesis. KITLG/SCF binding can activate several signaling pathways. Promotes phosphorylation of PIK3R1, the regulatory subunit of phosphatidylinositol 3-kinase, and subsequent activation of the kinase AKT1. KITLG/SCF and KIT also transmit signals via GRB2 and activation of RAS, RAF1 and the MAP kinases MAPK1/ERK2 and/or MAPK3/ERK1. KITLG/SCF and KIT promote activation of STAT family members STAT1, STAT3 and STAT5. KITLG/SCF and KIT promote activation of PLCG1, leading to the production of the cellular signaling molecules diacylglycerol and inositol 1,4,5-trisphosphate. KITLG/SCF acts synergistically with other cytokines, probably interleukins.
Post-translational A soluble form (sKITLG) is produced by proteolytic processing of isoform 1 in the extracellular domain. Found in two differentially glycosylated forms, LMW-SCF and HMW-SCF. LMW-SCF is fully N-glycosylated at Asn-145, partially N-glycosylated at Asn-90, O-glycosylated at Ser-167, Thr-168 and Thr-180, and not glycosylated at Asn-97 or Asn-118. HMW-SCF is N-glycosylated at Asn-118, Asn-90 and Asn-145, O-glycosylated at Ser-167, Thr-168 and Thr-180, and not glycosylated at Asn-97. A soluble form exists as a cleavage product of the extracellular domain. |
|---|