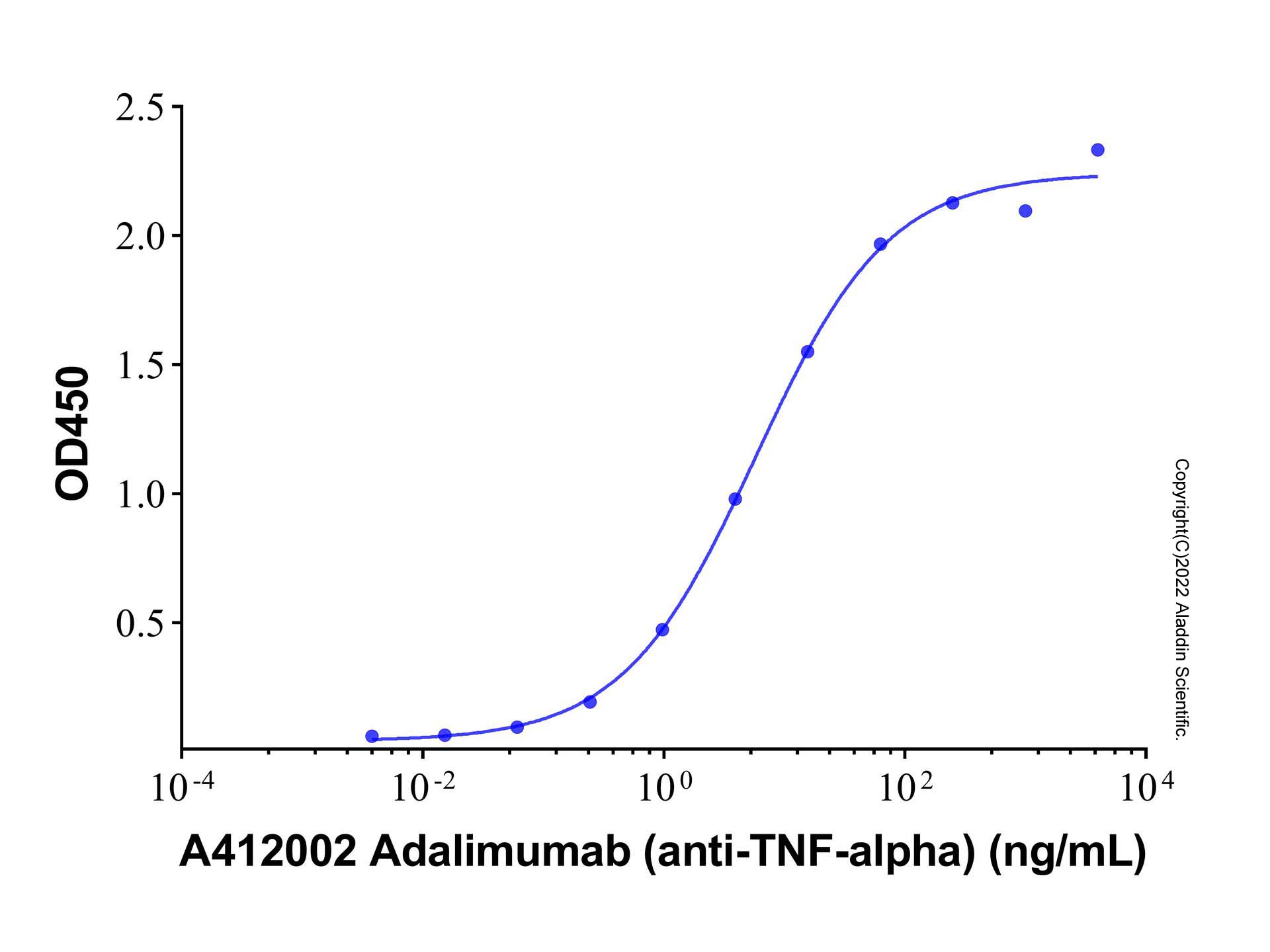
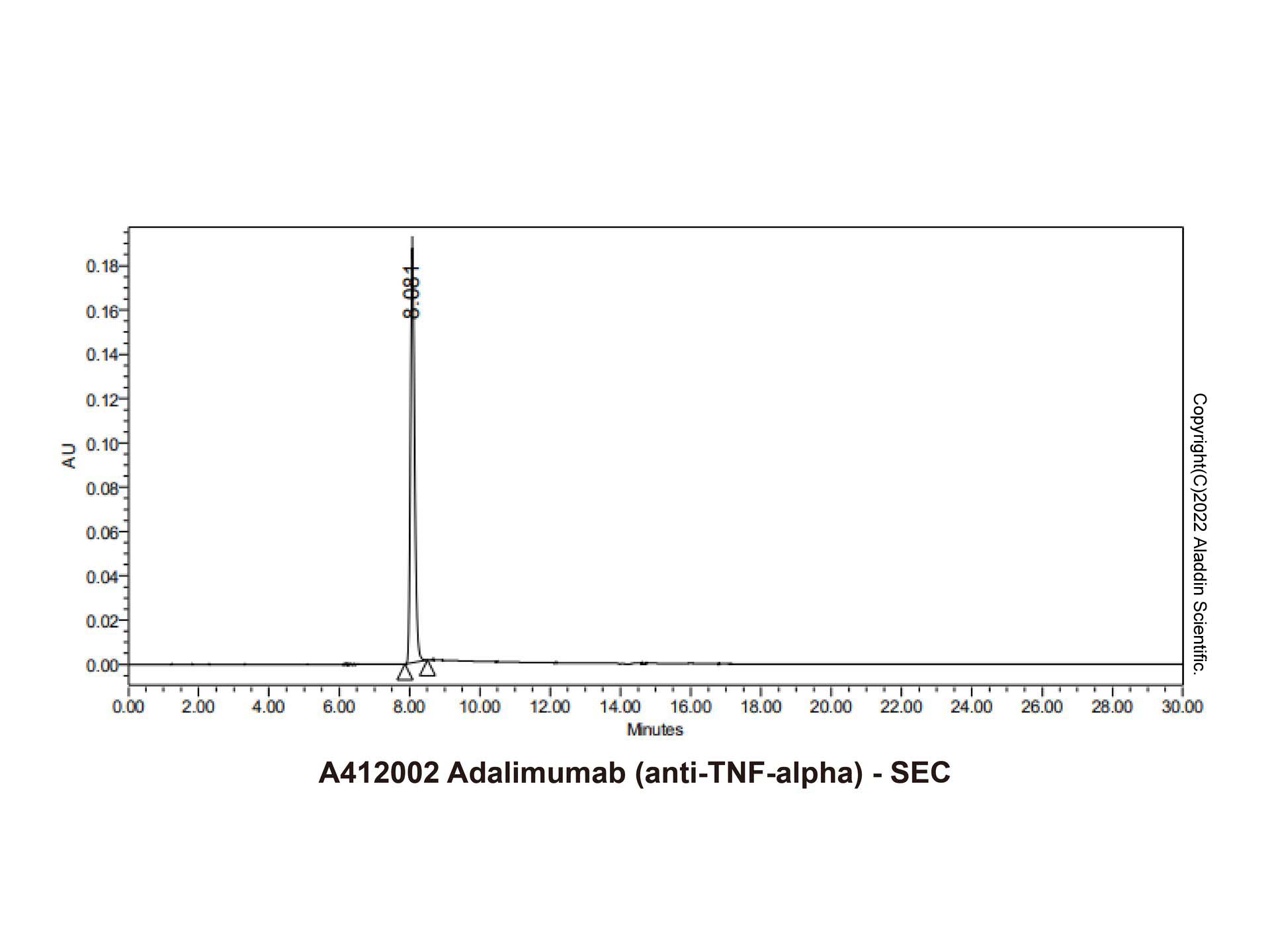
| 1. Kempeni J. (1999) Preliminary results of early clinical trials with the fully human anti-TNFalpha monoclonal antibody D2E7.. Ann Rheum Dis, 58 Suppl 1 (13): (I70-2). [PMID:10577977] [10.1021/op500134e] |
| 2. Wollheim FA. (2002) TNF inhibition as therapy for rheumatoid arthritis.. Expert Opin Investig Drugs, 11 (7): (947-53). [PMID:12084005] [10.1021/op500134e] |
| 3. Rau R. (2002) Adalimumab (a fully human anti-tumour necrosis factor alpha monoclonal antibody) in the treatment of active rheumatoid arthritis: the initial results of five trials.. Ann Rheum Dis, 61 Suppl 2 (13): (ii70-3). [PMID:12379628] [10.1021/op500134e] |
| 4. Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, Barisani-Asenbauer T, Franco P, Heiligenhaus A, Scales D et al.. (2016) Adalimumab in Patients with Active Noninfectious Uveitis.. N Engl J Med, 375 (10): (932-43). [PMID:27602665] [10.1021/op500134e] |
| 5. Kawalec P, Mikrut A, Wiśniewska N, Pilc A. (2013) Tumor necrosis factor-α antibodies (infliximab, adalimumab and certolizumab) in Crohn's disease: systematic review and meta-analysis.. Arch Med Sci, 9 (5): (765-779). [PMID:24273556] [10.1021/op500134e] |
| 6. Puri A, Niewiarowski A, Arai Y, Nomura H, Baird M, Dalrymple I, Warrington S, Boyce M. (2017) Pharmacokinetics, safety, tolerability and immunogenicity of FKB327, a new biosimilar medicine of adalimumab/Humira, in healthy subjects.. Br J Clin Pharmacol, 83 (7): (1405-1415). [PMID:28133772] [10.1021/op500134e] |
| 7. Jani RH, Gupta R, Bhatia G, Rathi G, Ashok Kumar P, Sharma R, Kumar U, Gauri LA, Jadhav P, Bartakke G et al.. (2016) A prospective, randomized, double-blind, multicentre, parallel-group, active controlled study to compare efficacy and safety of biosimilar adalimumab (Exemptia; ZRC-3197) and adalimumab (Humira) in patients with rheumatoid arthritis.. Int J Rheum Dis, 19 (11): (1157-1168). [PMID:26176644] [10.1021/op500134e] |
| 8. Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, Philipp S, Spelman L, Zhang N, Strober B. (2017) Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis.. Br J Dermatol, 177 (6): (1562-1574). [PMID:28755394] [10.1021/op500134e] |
| 9. Kronthaler U, Fritsch C, Hainzl O, Seidl A, da Silva A. (2018) Comparative functional and pharmacological characterization of Sandoz proposed biosimilar adalimumab (GP2017): rationale for extrapolation across indications.. Expert Opin Biol Ther, 18 (8): (921-930). [PMID:29962245] [10.1021/op500134e] |
| 10. Schreiber S, Yamamoto K, Muniz R, Iwura T. (2020) Physicochemical analysis and biological characterization of FKB327 as a biosimilar to adalimumab.. Pharmacol Res Perspect, 8 (3): (e00604). [PMID:32500668] [10.1021/op500134e] |