Determine the necessary mass, volume, or concentration for preparing a solution.
Cemiplimab (anti-PD-1) (C412010) - ELISA
Immobilized hu-PD-1 at 2 μg/mL can bind Cemiplimab (anti-PD-1) (C412010) with the EC₅₀ of 8.66 ng/mL.
| SKU | Size | Availability | Price | Qty |
|---|---|---|---|---|
| C412010-100μg | 100μg | Available within 8-12 weeks(?) Production requires sourcing of materials. We appreciate your patience and understanding. | $99.90 | |
| C412010-1mg | 1mg | In stock | $453.90 | |
| C412010-5mg | 5mg | In stock | $1,889.90 | |
| C412010-10mg | 10mg | Available within 8-12 weeks(?) Production requires sourcing of materials. We appreciate your patience and understanding. | $3,401.90 |
Purity>95% (SDS-PAGE&SEC); Endotoxin Level<1.0EU/mg; Human IgG4; CHO; ELISA, FACS, Functional assay, Animal Model; Unconjugated
| Product Name | Cemiplimab (anti-PD-1), for ELISA,Functional Assay,Kinetics (BLI),Kinetics (SPR),Flow Cyt various applications, specific to PDCD1, >95%, high purity, Human IgG4 |
|---|---|
| Synonyms | hPD-1 | Protein PD-1 |
| Specifications & Purity | ExactAb™, Validated, Carrier Free, Low Endotoxin, Azide Free, Recombinant, ≥95%(SDS-PAGE&SEC), Lot by Lot |
| Host species | Human |
| Specificity | PDCD1 |
| Application | ELISA,Functional Assay,Kinetics (BLI),Kinetics (SPR),Flow Cyt |
| Conjugation | Unconjugated |
| Grade | Azide Free, Carrier Free, ExactAb™, Low Endotoxin, Recombinant, Validated |
| Action Type | INHIBITOR |
| Mechanism of action | Antibody of programmed cell death 1 (CD279) |
| Product Description | Cemiplimab is a monoclonal IgG4 antibody with high affinity for programmed death receptor-1 (PD-1) that blocks PD-1/PD-L1 mediated T cell inhibition. It is commonly used in squamous cell skin cancer research. |
| Antibody Type | Primary antibody |
|---|---|
| Clonality | Recombinant |
| Isotype | Human IgG4 |
| Light Chain Type | kappa |
| SDS-PAGE | 26.0 kDa (Light Chain) & 49.9 kDa (Heavy Chain), under reducing conditions; 169.6 kDa, under non-reducing conditions. |
| Purification Method | Protein A purified |
| Purity | >95% |
| Source | CHO supernatant |
| Form | Liquid |
| Concentration | Lot by Lot |
| Storage Temp | Store at -80°C,Avoid repeated freezing and thawing |
| Shipped In | Ice chest + Ice pads |
| Stability And Storage | Store at -80℃ for 24 months. Upon delivery aliquot. Avoid freeze/thaw cycle. |
| CAS | 1801342-60-8 |
Cemiplimab (anti-PD-1) (C412010) - ELISA
Immobilized hu-PD-1 at 2 μg/mL can bind Cemiplimab (anti-PD-1) (C412010) with the EC₅₀ of 8.66 ng/mL.
Cemiplimab (anti-PD-1) (C412010) - SEC
The purity of Cemiplimab (anti-PD-1) (C412010) is more than 95% verified by HPLC.
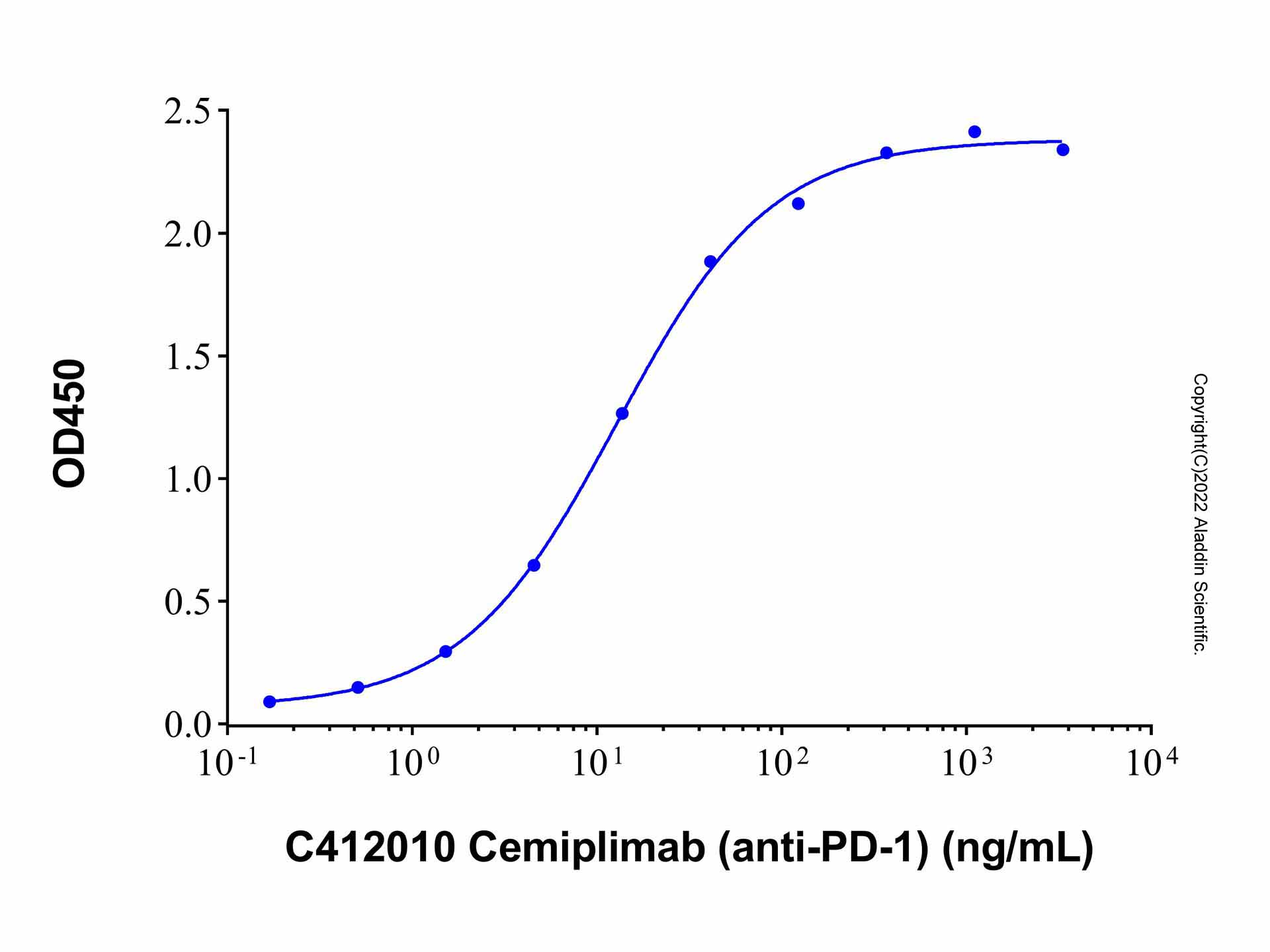
Cemiplimab (anti-PD-1) (C412010) - ELISA
Immobilized Recombinant Human PD-1 Protein (rp176241) at 1.0 μg/mL can bind Cemiplimab (anti-PD-1) (C412010) with the EC50 of 12.82 ng/mL.
| Activity Type | Activity Value -log(M) | Mechanism of Action | Activity Reference | Publications (PubMed IDs) |
|---|
| Activity Type | Relation | Activity value | Units | Action Type | Journal | PubMed Id | doi | Assay Aladdin ID |
|---|
| Mechanism of Action | Action Type | target ID | Target Name | Target Type | Target Organism | Binding Site Name | References |
|---|
| IMGT/mAb-DB | 846 |
|---|---|
| Reactome Reaction | R-HSA-9679421 |
| Reactome Drug | R-ALL-9679434 |
Enter Lot Number to search for COA:
Find and download the COA for your product by matching the lot number on the packaging.
| Lot Number | Certificate Type | Date | Item |
|---|---|---|---|
| Certificate of Analysis | Dec 20, 2023 | C412010 | |
| Certificate of Analysis | Dec 20, 2023 | C412010 |
| 1. Murphy AJ, Macdonald LE, Stevens S, Karow M, Dore AT, Pobursky K, Huang TT, Poueymirou WT, Esau L, Meola M et al.. (2014) Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice.. Proc Natl Acad Sci USA, 111 (14): (5153-8). [PMID:24706856] [10.1021/op500134e] |
| 2. Falchook GS, Leidner R, Stankevich E, Piening B, Bifulco C, Lowy I, Fury MG. (2016) Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN2810.. J Immunother Cancer, 4 (13): (70). [PMID:27879972] [10.1021/op500134e] |
| 3. Burova E, Hermann A, Waite J, Potocky T, Lai V, Hong S, Liu M, Allbritton O, Woodruff A, Wu Q et al.. (2017) Characterization of the Anti-PD-1 Antibody REGN2810 and Its Antitumor Activity in Human PD-1 Knock-In Mice.. Mol Cancer Ther, 16 (5): (861-870). [PMID:28265006] [10.1021/op500134e] |
| 4. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS et al.. (2018) PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma.. N Engl J Med, 379 (4): (341-351). [PMID:29863979] [10.1021/op500134e] |
| 5. Gerhard DS, Wagner L, Feingold EA, Shenmen CM, Grouse LH, Schuler G, Klein SL, Old S, Rasooly R, Good P et al.. (2004) The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC).. Genome Res, 14 (10B): (2121-7). [PMID:15489334] [10.1021/op500134e] |
| 6. Ota T, Suzuki Y, Nishikawa T, Otsuki T, Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K et al.. (2004) Complete sequencing and characterization of 21,243 full-length human cDNAs.. Nat Genet, 36 (1): (40-5). [PMID:14702039] [10.1021/op500134e] |
| 7. Kline J, Gajewski TF. (2010) Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer.. Curr Opin Investig Drugs, 11 (12): (1354-9). [PMID:21154117] [10.1021/op500134e] |
| 8. Hall RD, Gray JE, Chiappori AA. (2013) Beyond the standard of care: a review of novel immunotherapy trials for the treatment of lung cancer.. Cancer Control, 20 (1): (22-31). [PMID:23302904] [10.1021/op500134e] |
| 9. Zak KM, Kitel R, Przetocka S, Golik P, Guzik K, Musielak B, Dömling A, Dubin G, Holak TA. (2015) Structure of the Complex of Human Programmed Death 1, PD-1, and Its Ligand PD-L1.. Structure, 23 (12): (2341-2348). [PMID:26602187] [10.1021/op500134e] |
| 10. L R Finger,J Pu,R Wasserman,R Vibhakar,E Louie,R R Hardy,P D Burrows,L G Billips. (1997-10-23) The human PD-1 gene: complete cDNA, genomic organization, and developmentally regulated expression in B cell progenitors.. Gene, 197 ((1-2)): (177-187). [PMID:9332365] |
| 11. Cheng B, Ren Y, Niu X, Wang W, Wang S, Tu Y, Liu S, Wang J, Yang D, Liao G, Chen J.. (2020) Discovery of Novel Resorcinol Dibenzyl Ethers Targeting the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Interaction as Potential Anticancer Agents.. J Med Chem, 63 (15): (8338-8358). [PMID:32667799] [10.1021/acs.jmedchem.0c00574] |
| 12. Shinohara, T T, Taniwaki, M M, Ishida, Y Y, Kawaichi, M M and Honjo, T T.. (1994) Structure and chromosomal localization of the human PD-1 gene (PDCD1).. Genomics, [PMID:7851902] |
| 13. Prokunina, Ludmila L and 23 more authors.. (2002) A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans.. Nature genetics, [PMID:12402038] |
| 14. Fife, Brian T BT and Pauken, Kristen E KE.. (2011) The role of the PD-1 pathway in autoimmunity and peripheral tolerance.. Annals of the New York Academy of Sciences, [PMID:21276005] |
| 15. Topalian, Suzanne L SL and 29 more authors.. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer.. The New England journal of medicine, (28): [PMID:22658127] |
| 16. Cheng, Xiaoxiao X and 18 more authors.. (2013) Structure and interactions of the human programmed cell death 1 receptor.. The Journal of biological chemistry, (26): [PMID:23417675] |
| 17. Robert, Caroline C and 27 more authors.. (2015) Nivolumab in previously untreated melanoma without BRAF mutation.. The New England journal of medicine, (22): [PMID:25399552] |
| 18. McDermott, J J and Jimeno, A A.. (2015) Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer.. Drugs of today (Barcelona, Spain : 1998), [PMID:25685857] |
| 19. Na, Zhenkun and 6 more authors.. (2017) Structural basis for blocking PD-1-mediated immune suppression by therapeutic antibody pembrolizumab.. Cell research, [PMID:27325296] |
| 20. Pascolutti, Roberta R and 6 more authors.. (2016) Structure and Dynamics of PD-L1 and an Ultra-High-Affinity PD-1 Receptor Mutant.. Structure (London, England : 1993), (4): [PMID:27618663] |
| 21. Horita, Shoichiro S and 5 more authors.. (2016) High-resolution crystal structure of the therapeutic antibody pembrolizumab bound to the human PD-1.. Scientific reports, (13): [PMID:27734966] |
| 22. Tan, Shuguang S and 15 more authors.. (2017) An unexpected N-terminal loop in PD-1 dominates binding by nivolumab.. Nature communications, (6): [PMID:28165004] |
| 23. Berger, Kristin Nicole KN and Pu, Jeffrey Jiayu JJ.. (2018) PD-1 pathway and its clinical application: A 20year journey after discovery of the complete human PD-1 gene.. Gene, (5): [PMID:28951311] |