Determine the necessary mass, volume, or concentration for preparing a solution.
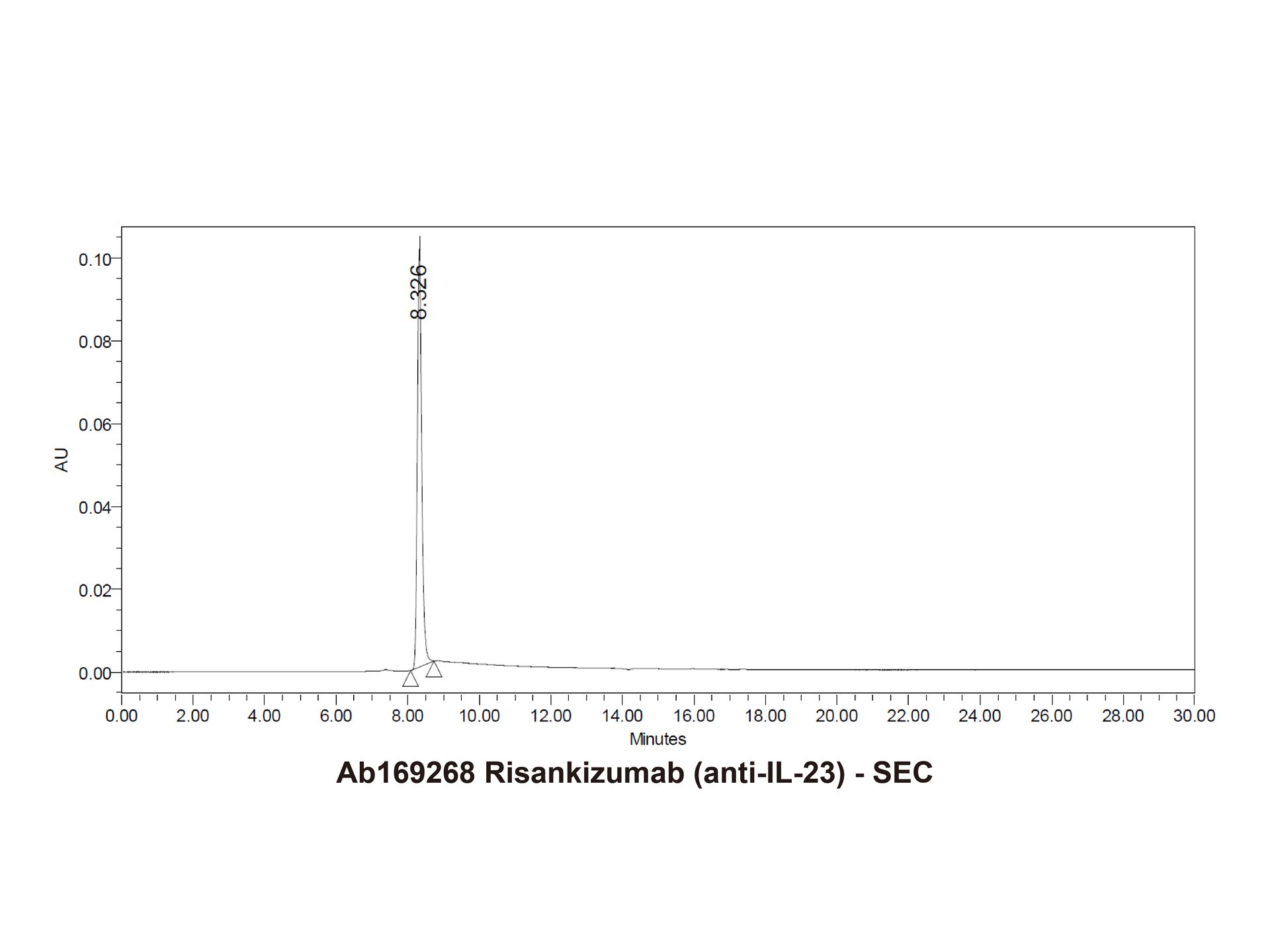
Risankizumab (anti-IL-23) (Ab169268) - SEC
The purity of Risankizumab (anti-IL-23) (Ab169268) is more than 95% verified by HPLC.
| SKU | Size | Availability | Price | Qty |
|---|---|---|---|---|
| Ab169268-100μg | 100μg | Available within 8-12 weeks(?) Production requires sourcing of materials. We appreciate your patience and understanding. | $139.90 | |
| Ab169268-1mg | 1mg | Available within 4-8 weeks(?) Items will be manufactured post-order and can take 4-8 weeks. Thank you for your patience! | $657.90 | |
| Ab169268-5mg | 5mg | Available within 4-8 weeks(?) Items will be manufactured post-order and can take 4-8 weeks. Thank you for your patience! | $1,407.90 | |
| Ab169268-10mg | 10mg | Available within 8-12 weeks(?) Production requires sourcing of materials. We appreciate your patience and understanding. | $2,457.90 |
Purity>95% (SDS-PAGE&SEC); Endotoxin Level<1.0EU/mg; Human IgG1; CHO; ELISA, FACS, Functional assay, Animal Model; Unconjugated
| Product Name | Risankizumab (anti-IL-23) - Primary antibody, for ELISA,Functional Assay,Flow cytometry,Kinetics (BLI),Kinetics (SPR) various applications, specific to IL23A, >95%, high purity, Human IgG1 |
|---|---|
| Synonyms | Recombinant Risankizumab Antibody | Risankizumab | Risankizumab rzaa | BI 655066 | Risankizumab (anti-IL-23) | Anti-IL-23a Reference Antibody (risankizumab) | IL 23 A antibody | IL 23 antibody | IL 23 subunit alpha antibody | IL 23A antibody | IL 23p19 an |
| Specifications & Purity | Carrier Free, Recombinant, ExactAb™, Low Endotoxin, Azide Free, Validated, ≥95%(SDS-PAGE&SEC-HPLC), See COA |
| Host species | Human |
| Specificity | IL23A |
| Application | ELISA,Functional Assay,Flow cytometry,Kinetics (BLI),Kinetics (SPR) |
| Conjugation | Unconjugated |
| Grade | Azide Free, Carrier Free, ExactAb™, Low Endotoxin, Recombinant, Validated |
| Action Type | INHIBITOR |
| Mechanism of action | INHIBITOR of Interleukin-23 inhibitor |
| Product Description | Risankizumab targets the p19 subunit of IL-23. Risankizumab is indicated for the treatment of psoriasis and is safe and effective in maintaining remission in active Crohn's disease. |
| Antibody Type | Primary antibody |
|---|---|
| Clonality | Recombinant |
| Isotype | Human IgG1 |
| Light Chain Type | kappa |
| SDS-PAGE | 26.1 kDa (Light Chain) & 51.0 kDa (Heavy Chain), under reducing conditions; 166.7 kDa, under non-reducing conditions. |
| Purification Method | Protein A purified |
| Purity | >95% |
| Source | CHO supernatant |
| Form | Liquid |
| Concentration | See COA |
| Storage Temp | Store at -80°C,Avoid repeated freezing and thawing |
| Shipped In | Ice chest + Ice pads |
| Stability And Storage | Store at -80℃ for 24 months. Upon delivery aliquot. Avoid freeze/thaw cycle. |
| CAS | 1612838-76-2 |

Risankizumab (anti-IL-23) (Ab169268) - SEC
The purity of Risankizumab (anti-IL-23) (Ab169268) is more than 95% verified by HPLC.
| Activity Type | Activity Value -log(M) | Mechanism of Action | Activity Reference | Publications (PubMed IDs) |
|---|
Find and download the COA for your product by matching the lot number on the packaging.
2 results found
| Lot Number | Certificate Type | Date | Item |
|---|---|---|---|
| Certificate of Analysis | Sep 14, 2023 | Ab169268 | |
| Certificate of Analysis | Sep 14, 2023 | Ab169268 |
| 1. Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, Timms K, Gutin A, Abkevic V, Burden AD et al.. (2007) Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis.. Hum Genet, 122 (2): (201-6). [PMID:17587057] [10.1021/op500134e] |
| 2. Elmaagacli AH, Koldehoff M, Landt O, Beelen DW. (2008) Relation of an interleukin-23 receptor gene polymorphism to graft-versus-host disease after hematopoietic-cell transplantation.. Bone Marrow Transplant, 41 (9): (821-6). [PMID:18209723] [10.1021/op500134e] |
| 3. McGovern D, Powrie F. (2007) The IL23 axis plays a key role in the pathogenesis of IBD.. Gut, 56 (10): (1333-6). [PMID:17872562] [10.1021/op500134e] |
| 4. Toussirot E. (2012) The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases.. Inflamm Allergy Drug Targets, 11 (2): (159-68). [PMID:22280236] [10.1021/op500134e] |
| 5. Baeten DL, Kuchroo VK. (2013) How Cytokine networks fuel inflammation: Interleukin-17 and a tale of two autoimmune diseases.. Nat Med, 19 (7): (824-5). [PMID:23836225] [10.1021/op500134e] |
| 6. Gaffen SL, Jain R, Garg AV, Cua DJ. (2014) The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing.. Nat Rev Immunol, 14 (9): (585-600). [PMID:25145755] [10.1021/op500134e] |
| 7. Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, Lalovic B, Aslanyan S, Wang EE, Hall D et al.. (2015) Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial.. J Allergy Clin Immunol, 136 (1): (116-124.e7). [PMID:25769911] [10.1021/op500134e] |
| 8. Singh S, Kroe-Barrett RR, Canada KA, Zhu X, Sepulveda E, Wu H, He Y, Raymond EL, Ahlberg J, Frego LE et al.. (2015) Selective targeting of the IL23 pathway: Generation and characterization of a novel high-affinity humanized anti-IL23A antibody.. MAbs, 7 (4): (778-91). [PMID:25905918] [10.1021/op500134e] |
| 9. Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, Papp KA, Sofen H, Puig L, Foley P et al.. (2018) Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials.. Lancet, 392 (10148): (650-661). [PMID:30097359] [10.1021/op500134e] |
| 10. Al-Janabi A, Jabbar-Lopez ZK, Griffiths CEM, Yiu ZZN. (2019) Risankizumab vs. ustekinumab for plaque psoriasis: a critical appraisal.. Br J Dermatol, 180 (6): (1348-1351). [PMID:30632140] [10.1021/op500134e] |
| 11. Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F et al.. (2002) A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R.. J Immunol, 168 (11): (5699-708). [PMID:12023369] [10.1021/op500134e] |
| 12. Campa M, Mansouri B, Warren R, Menter A. (2016) A Review of Biologic Therapies Targeting IL-23 and IL-17 for Use in Moderate-to-Severe Plaque Psoriasis.. Dermatol Ther (Heidelb), 6 (1): (1-12). [PMID:26714681] [10.1021/op500134e] |
| 13. Oppmann, B B and 24 more authors.. (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12.. Immunity, [PMID:11114383] |
| 14. Pirhonen, Jaana J, Matikainen, Sampsa S and Julkunen, Ilkka I.. (2002) Regulation of virus-induced IL-12 and IL-23 expression in human macrophages.. Journal of immunology (Baltimore, Md. : 1950), (15): [PMID:12421946] |
| 15. Smits, Hermelijn H HH and 8 more authors.. (2004) Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development.. European journal of immunology, [PMID:15114670] |
| 16. Schnurr, Max M and 5 more authors.. (2005) Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway.. Blood, (15): [PMID:15486065] |
| 17. Fedele, Giorgio G and 5 more authors.. (2005) Bordetella pertussis-infected human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization and interleukin-23 expression.. Infection and immunity, [PMID:15731058] |
| 18. Vanden Eijnden, Serge S, Goriely, Stanislas S, De Wit, Dominique D, Goldman, Michel M and Willems, Fabienne F.. (2006) Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns.. European journal of immunology, [PMID:16342235] |
| 19. Piskin, Gamze G, Sylva-Steenland, Regien M R RM, Bos, Jan D JD and Teunissen, Marcel B M MB.. (2006) In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin.. Journal of immunology (Baltimore, Md. : 1950), (1): [PMID:16424222] |
| 20. Langowski, John L JL and 9 more authors.. (2006) IL-23 promotes tumour incidence and growth.. Nature, (27): [PMID:16688182] |
| 21. Vaknin-Dembinsky, Adi A, Balashov, Konstantin K and Weiner, Howard L HL.. (2006) IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production.. Journal of immunology (Baltimore, Md. : 1950), (15): [PMID:16751425] |
| 22. Wilson, Nicholas J NJ and 15 more authors.. (2007) Development, cytokine profile and function of human interleukin 17-producing helper T cells.. Nature immunology, [PMID:17676044] |
| 23. Lupardus, Patrick J PJ and Garcia, K Christopher KC.. (2008) The structure of interleukin-23 reveals the molecular basis of p40 subunit sharing with interleukin-12.. Journal of molecular biology, (17): [PMID:18680750] |
| 24. Papp, K K and 12 more authors.. (2015) Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial.. The British journal of dermatology, [PMID:26042589] |
| 25. Bloch, Yehudi and 11 more authors.. (2018) Structural Activation of Pro-inflammatory Human Cytokine IL-23 by Cognate IL-23 Receptor Enables Recruitment of the Shared Receptor IL-12Rβ1.. Immunity, (16): [PMID:29287995] |
| 26. Haugh, Isabel M; Preston, Allie K; Kivelevitch, Dario N and Menter, Alan M.. (2018) Risankizumab: an anti-IL-23 antibody for the treatment of psoriasis.. Drug design, development and therapy, [PMID:30518998] |
| 27. Machado, Álvaro Á and Torres, Tiago T.. (2018) Spotlight on risankizumab and its potential in the treatment of plaque psoriasis: evidence to date.. Psoriasis (Auckland, N.Z.), [PMID:30519540] |
| 28. Sun, Rui and Abraham, Clara.. (2020) IL23 Promotes Antimicrobial Pathways in Human Macrophages, Which Are Reduced With the IBD-Protective IL23R R381Q Variant.. Cellular and molecular gastroenterology and hepatology, [PMID:32474165] |
| 29. Glassman, Caleb R and 10 more authors.. (2021) Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells.. Cell, (18): [PMID:33606986] |